Destination Unknown: Durable Responses in Higher-Risk Myelodysplastic Syndrome
Theo de Witte
Higher-risk MDS is a collective term based on the Revised International Prognostic Scoring System (IPSS-R) that encompasses: intermediate-risk patients with a score ≥3.5, high-risk, and very high-risk patients.7,8 Studies have demonstrated that patients with the highest IPSS-R risk disease have the shortest survival duration, with a clear distinction in OS and hazard ratio for premature death between higher-risk MDS and other low- or intermediate-risk groups, emphasised de Witte.7,8 Even after allo-HCT, the IPSS-R cytogenetic risk classification continues to have an impact on OS, driven largely by the higher cumulative incidence of relapse in those with poor- or very poor-risk cytogenetic features.9
According to the current management algorithms, treatment for higher-risk MDS is centred around two main approaches: allo-HCT for fit patients with good performance status and non-transplant strategies for the remainder.10 Allo-HCT is increasingly used as the only curative treatment and, depending on MDS status and risk levels, 25–75% of transplant recipients can attain durable disease freedom and establish long-term survival.10,11 However, less than 15% of patients with higher-risk MDS are suitable candidates for haematopoietic stem cell transplantation (HSCT) and HMAs remain the current SOC for these patients who are transplant-ineligible.10,12,13 For eligible patients, the decision of whether to go direct to transplant or pursue cytoreductive therapy first is mainly based on bone marrow blast levels (<10% or ≥10%).10 Patients with higher-risk MDS benefit from early transplantation as the interval between diagnosis and allo-HCT is one of the major factors that dictates outcomes: “The sooner the better,” stressed de Witte.11 Looking to the future, new non-transplant modalities, including HMAs with or without investigational drugs, may influence the indication, timing, and preparation for HSCT.10
Retrospective studies have also demonstrated improved outcomes for patients with MDS transplanted in complete remission (CR) compared to those with active disease at the time of allo-HCT. For example, a large study of 513 patients with chronic myelomonocytic leukaemia from the European Group for Blood and Marrow Transplantation (EBMT) registry showed that CR prior to transplant was the only prognostic factor for OS (p=0.005).14 Smaller studies have also addressed the role of preventive treatment after allo-HCT in preventing relapse. In a study of 28 patients with AML who commenced therapy with AZA approximately 9 months after transplantation, induction of a cytotoxic T cell response was found to be associated with improved relapse-free survival (hazard ratio: 0.29; p=0.02).15 In a similar single-arm study of 30 patients treated with the oral formulation of AZA (CC-486), median OS was not reached and only six out of 28 evaluable patients had relapsed or progressed.16 In contrast, in a genetically randomised study of 162 patients, bridging with AZA to allo-HCT was associated with a considerable rate of dropouts because of progression, mortality, and adverse events (AEs).17 The potential of cytoreductive therapy, as well as AZA used prior to allo-HCT, needs to be confirmed in larger, randomised controlled trials, noted de Witte.
For over a decade, HMAs have been the standard choice for treating the vast majority of patients with higher-risk MDS who are not eligible for stem cell transplant.12 However, half of treated patients fail to respond to HMAs and CR rates are consistently low, at less than 20%.6,18-23 Duration of remission is also limited, with most patients with higher-risk MDS losing response to HMAs within 11–15 months and progressing to AML in less than 2 years on average.19-21,24 OS outcomes after HMA failure are unequivocally poor, with a median OS of just 4–6 months in patients with resistance to or prior failure on HMAs.25,26
Unsurprisingly, the overall contribution of HMAs to the prolongation of life in patients with higher-risk MDS remains limited and unconfirmed in real-world studies.12 A lack of progress to date in higher-risk MDS is clearly illustrated in a recent analysis of Surveillance, Epidemiology, and End Results (SEER) data, which shows no improvement in 2-year survival probability from 2001–2004 (before the availability of HMAs) compared with 2007–2013 (after the introduction of HMAs) across all age groups.12,27
The rarity of CRs and durable responses with HMAs, as well as the limited treatment options following failure, underscores the urgent, unmet need for new therapies for patients diagnosed with higher-risk MDS who are not eligible for transplant. As a result, several new iterations of old HMAs, including oral formulations, have undergone investigation in clinical trials. These include guadecitabine, a novel HMA with extended decitabine exposure.28 Guadecitabine was evaluated in a (now completed) Phase II study as a second-line treatment in 56 patients with high-risk MDS; here, two patients achieved CR and three survived for more than 2 years.28 Given the lack of significant clinical advantage, the ongoing development of guadecitabine has been stopped. An oral combination of decitabine and cedazuridine (ASTX727) achieved an exposure similar to that of intravenous (IV) decitabine at 20 mg/m2 in a Phase I/II study. The subsequent Phase III trial in 138 patients with high-risk MDS or chronic myelomonocytic leukaemia demonstrated equivalency of oral versus IV ASTX727 pharmacokinetics.29 ASTX727 has been now approved by the U.S. Food and Drug Administration (FDA) for the treatment of all subtypes of MDS. Finally, CC-486, an unmodified oral AZA, showed 17% bioavailability compared with a single dose of IV AZA tested in AML as post-remission maintenance, leading to improved OS compared with placebo-treated controls.16 In general, we can say that the outcomes with these drugs might be equivalent, but not better than the original parenteral formulation, noted de Witte. Beyond HMAs, the field is also evolving rapidly and a large number of new drugs targeting different disease pathways are undergoing clinical development for higher-risk MDS, either alone or in combination with existing HMAs. These include the B cell leukaemia 2 (BCL-2) inhibitor venetoclax, and the anti-T cell Ig domain and mucin domain 3 (TIM-3) IgG4 antibody, sabatolimab.
Summing up, de Witte reiterated that allo-HCT currently remains the only treatment for higher-risk MDS that is potentially curative. The SOC for most patients who are ineligible for transplant is HMA therapy, which is associated with unsatisfactory rates of CR and a relatively short duration of response (DOR). Therefore, there is clearly an unmet need for new treatments, such as investigational drugs in combination with HMAs, that can provide durable responses and improve outcomes in patients with higher-risk MDS who are ineligible for stem cell transplantation, he concluded.
Navigating Higher-Risk Myelodysplastic Syndrome Treatment with Available Therapies
Guillermo Sanz and Panel
Sanz outlined two hypothetical clinical case studies that reflect the limited treatment options currently available to patients with IPSS-R higher-risk MDS (Table 1).
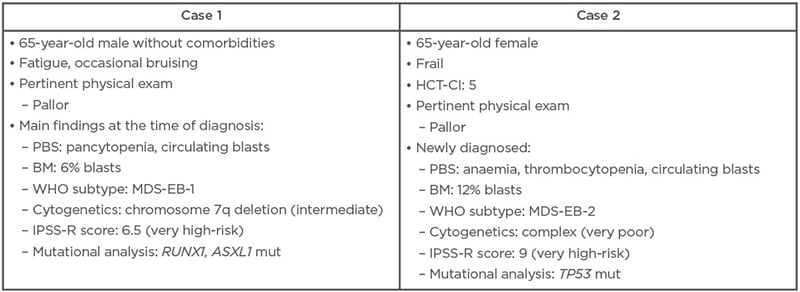
Table 1: Two hypothetical clinical cases of higher-risk myelodysplastic syndrome.
BM: bone marrow; HCT-CI: haematopoietic cell transplantation-specific comorbidity index; IPSS-R: Revised International Prognostic Scoring System; MDS-EB: myelodysplastic syndrome with excess blasts; mut: mutated; PBS: peripheral blood smear; WHO: World Health Organization.
Given the fitness and good health status of the first case study, the panel agreed that allo-HCT was the optimal treatment choice assuming that a suitable donor could be identified. HSCT is the only potentially curative treatment for higher-risk MDS and provides the best chance of achieving durable responses with associated improvements in survival and quality of life. It was suggested by de Witte that transplant should be undertaken as early as possible, with no induction treatment required due to the patient’s low blast count.
For the second clinical case, the panel agreed that, based on the patient’s frailty and comorbidities, this higher-risk MDS was unlikely to be a good candidate for HSCT or intensive chemotherapy, so the patient would usually receive HMA therapy instead. However, given the patient’s complex karyotype, enrolment in a clinical trial of investigational therapy was actually seen as the preferred option over HMAs, as this would yield the greatest change of achieving a durable response. TP53-mutated patients treated with AZA generally have a poor prognosis, emphasised Sanz, and durable responses are rare.
In all cases, when managing higher-risk MDS, the panel stressed the importance of effectively communicating the risk–benefit ratio of different approaches and considering the patient’s preference on treatment choice.
Sanz highlighted that there are several points in the course of disease management when patients with higher-risk MDS could be considered for enrolment in clinical trials. For older, transplant-eligible patients with lower blast percentages and intermediate- or poor-risk cytogenetics, this includes the time period immediately prior to allo-HCT and/or as an ongoing therapy in cases where a suitable donor cannot be identified. Participation in a clinical trial with novel agent(s) is also recommended for transplant-ineligible patients who fail to respond to an adequate course (≥6 cycles) of HMA therapy.
The Path to Long-Term Remission: New Data from Emerging Pathways in Higher-Risk Myelodysplastic Syndrome
Valeria Santini
For patients with higher-risk MDS who are ineligible for allo-HCT, or where no donor is available, the SOC treatment is with HMAs. However, enrolment into a clinical trial of investigational agents plus HMAs should also be considered, said Santini, and investigational agents should constitute an important option for patients who do not respond, relapse, or progress on HMAs (although no such trials are ongoing at present).30 It can take “some time” to attain a response to HMAs, Santini expanded, and interruption of treatment for any reason (e.g., lack of compliance) can provoke a loss of response.31 In addition, patients with complex karyotypes often fail to achieve durable responses to this SOC treatment.18,32 Once a patient has relapsed or become resistant to HMAs, survival is extremely short, irrespective of further treatment, and mechanisms underlying primary and secondary resistance to HMAs continue to remain elusive.26,33,34 In general, the more we discover about altered cellular pathways and different somatic mutations in MDS, the more we can target them, explained Santini. To this end, a number of promising new approaches are currently undergoing clinical investigation with the aim of optimising treatment in patients with higher-risk MDS (Figure 1).35-38
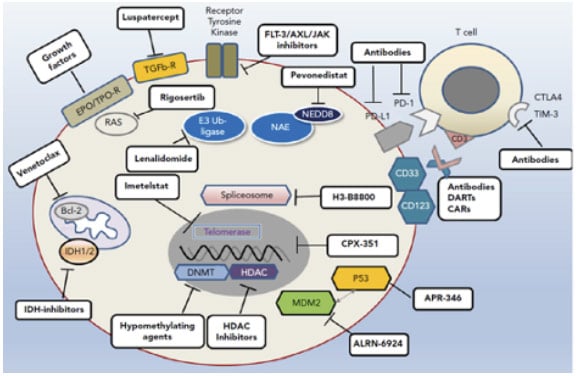
Figure 1: Avenues of therapeutic exploration in higher-risk myelodysplastic syndrome.35
AXL: anexelekto; BCL-2: B cell lymphoma 2; CAR: chimeric antigen receptor; CD: cluster of differentiation; CTLA-4: cytotoxic T-lymphocyte-associated protein 4; DART: dual-affinity retargeting antibody; EPO: erythropoietin; FLT-3: fms-like tyrosine kinase 3; HDAC: histone deacetylases; IDH: isocitrate dehydrogenase; MDM2: mouse double minute 2; NEDD-8: neuronal precursor cell-expressed developmentally down-regulated protein 8; PD-1: programmed cell death protein 1; PD-L1: programmed death-ligand 1; TIM-3: T cell immunoglobulin domain and mucin domain 3; TPO-R: thrombopoietin receptor.
Adapted from Platzbecker.35
Pevonedistat is an inhibitor of the NEDD-8 activating enzyme that causes an accumulation of tumour-suppressive substrates within cells, ultimately leading to senescence, apoptosis, and autophagy of neoplastic MDS cells.39-41 In a Phase II trial (n=59) in higher-risk MDS, pevonedistat plus AZA increased overall response rate (ORR), DOR, and event-free survival when compared to AZA alone.42 ORR rates were 79% versus 57%, with CR rates of 52% versus 27%, and the median DOR was 34.6 months versus 13.1 months, respectively.42 We are not used to seeing CR rates above 50% and this long DOR with AZA used as a single drug, noted Santini. Pevonedistat plus AZA was well-tolerated, with a safety profile comparable to AZA used alone.42 Although these Phase II data were notable, these findings were not confirmed by the Phase III PANTHER trial (reported after the symposium), which failed to meet the primary endpoint of improved event-free survival.43 A detailed analysis of the results from this trial is currently underway, but further development of pevonedistat has been stopped.
Also under investigation in MDS is venetoclax, a well-known oral small-molecule inhibitor of BCL-2 with proven activity in AML. Venetoclax selectively targets the regulatory protein BCL-2 that is overexpressed in cancer cells, triggering apoptosis.44-46 Venetoclax plus AZA produced an ORR of 79.0%, with CR and marrow CR rates of 39.7% each in a Phase Ib study (n=78) in higher-risk MDS.47 Responses lasted around 1 year, noted Santini, but we still need to see what the OS of these patients will be with longer follow-up. Myelosuppression was greater than that seen with AZA alone and substantial neutropenia led to dose reductions and delay of subsequent courses of therapy.47
Magrolimab is an anti-cluster of differentiation 47 (CD47) monoclonal antibody that abolishes the ‘do not eat me’ signal in myeloid malignancies, allowing for phagocytosis of leukaemic and neoplastic cells by macrophages, thereby targeting microenvironment involvement.48 Magrolimab plus AZA achieved promising ORR and durable response, as well as improved OS, in patients with higher-risk MDS in a Phase Ib trial.49 The ORR was 91% and CR rate was 42% in patients with higher-risk MDS. Responses also deepened over time, with a 6-month CR rate of 56%. Haematologic toxicity was not greater than expected for AZA alone and no patient had to stop treatment due to drug-related AEs.49 The Phase III ENHANCE study of magrolimab is currently underway.50
Also in the area of immune-myeloid therapy, sabatolimab is a novel high-affinity, humanised, IgG4 monoclonal antibody targeting TIM-3, which is under clinical development to treat higher-risk MDS.51,52 TIM-3 is an inhibitory antigen receptor found on immune cells and also highly expressed on leukaemic stem cells (LSC) and MDS blasts, but not haematopoietic stem cells, making it an attractive therapeutic target.52-55 By binding to TIM-3, sabatolimab “reawakens” the immune system to enable direct and selective attack of leukaemic cells, explained Santini.54 Sabatolimab also enhances antibody-dependent cellular phagocytosis, facilitating immune cell-mediated killing of LSCs/blasts, and inhibits LSC self-renewal.54 In a Phase Ib study, sabatolimab in combination with HMA demonstrated promising durable clinical benefit in patients with high- and very high-risk MDS (Figure 2).
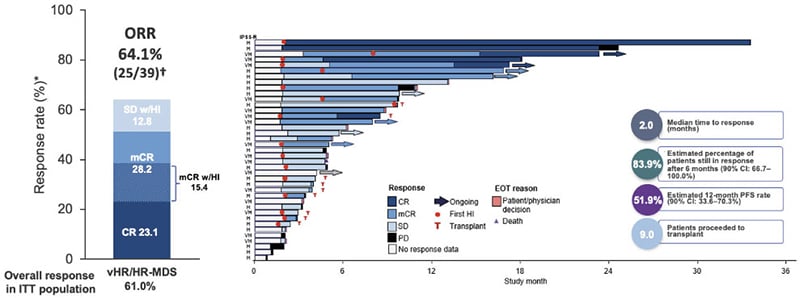
Figure 2: Durable clinical responses with sabatolimab plus hypomethylating agents in higher-risk myelodysplastic syndrome in a Phase Ib study.37
*Evaluable patients, including patients with a valid baseline and at least one post-baseline bone marrow assessment or if they had disease progression or disease-related death prior to the first marrow assessment.
†ORR for patients, including patients with MDS or CMML was defined as CR + mCR + PR + SE with HI.
CI: confidence interval; CMML: chronic myelomonocytic leukaemia; CR: complete remission; CRi: complete remission with incomplete haematological recovery; EOT: end of treatment; HI: haematological improvement; HR: high-risk; IPSS-R: Revised International Prognostic Scoring System; ITT: intent-to-treat; mCR: bone marrow complete remission; MDS: myelodysplastic syndrome; ORR: overall response rate; PD: progressive disease; PFS: progression-free survival; PR: partial remission; SD: stable disease; vHR, very high-risk.
Adapted from Brunner et al.37
The ORR was 64%, again with the “important” increases in CR (CR: 23%; bone marrow CR: 28%), noted Santini.37 In patients carrying mutations that are markers of poor prognosis, the remission rate remained consistently above 50% (55% with the TP53 mutations and 59% in patients with ≥1 of TP53, RUNX1, or ASXL1 mutations).56 Sabatolimab plus HMA also showed activity in elderly patients, with similar remission rates observed in those aged ≥75 years old (50%) and 65–74 years old (65%).56 Santini described the DOR with the combination of sabatolimab and HMA as “extremely good,” with more than 80% of patients still in response after 6 months.37 Overall, sabatolimab plus HMA proved safe and well-tolerated, with a safety profile similar to HMAs alone. There were no discontinuations due to AEs and no Grade ≥3 immune-mediated adverse events.37 Note that updated data have been presented at European Hematology Association (EHA) Congress 202157 and American Society of Hematology (ASH) Annual Meeting 2021.58 These results constitute a completely new approach to treating MDS, remarked Santini, an antibody targeting two different populations: the active immune population and the leukaemic dysplastic clones.
A number of specific mutation-driven investigational therapies are also under evaluation in higher-risk MDS. Eprenetapopt (APR-246) is a tumour protein-53 reactivator in clinical development for TP53-mutated MDS and AML. TP53 mutations are present in approximately 20% of patients with MDS and are associated with other high-risk features and worse clinical outcomes.59-61 Eprenetapopt restores TP53 to its wild-type conformation, thereby reactivating the mutated TP53 protein within tumour cells, leading to apoptosis.59-61 Unfortunately, the combination of eprenetapopt and AZA failed to meet the primary endpoint in a recent Phase III study, with CR rates for the combination (33%) not significantly different to AZA alone (22%; p=0.13).62 Median OS was also similar between the two arms of the study. Other gene-specific agents under investigation in higher-risk MDS include those targeting IDH1/IDH2 mutations that, although not frequently occurring, confer a negative prognosis.
Ivosidenib and enasidenib are oral drugs, known to be active in AML, which reverse the mutant IDH1/IDH2-mediated block of differentiation.63 In a Phase I trial (n=12) of ivosidenib, ORR in patients with MDS carrying the IDH1 mutation was 75% (CR: 42%), even though the majority of patients (nine out of 12) had received prior HMAs and relapsed.64 Similarly, in a Phase II, multicentre, open-label study (n=31) of enasidenib in patients with high-risk IDH2-mutated MDS, ORR was 56% in the subgroup of patients with prior HMA failure and 85% in treatment-naïve patients who received enasidenib in combination with AZA.65 However, Santini noted that these investigational therapies have only been evaluated in patients carrying the specific mutation of interest.
Given the breadth of novel agents under development for higher-risk MDS, with different modes of action targeting different disease pathways, potential future combination treatment strategies could include HMAs plus one or more investigational therapies in doublet or triplet combination regimens. Moving forward, it may even be possible to eliminate the HMA backbone entirely, postulated Santini, by identifying the optimal combination of investigational therapies. With multiple new treatment pathways under exploration, and the potential for novel combination therapies, a therapeutic revolution may be underway that will ultimately improve treatment outcomes for patients with higher-risk MDS who are ineligible for transplant.
Future Perspectives: Innovations In Situ
Panel
Looking to the future, investigational therapies in clinical development for higher-risk MDS offer a compelling opportunity to optimise therapeutic choices and even select different treatments for specific patients. One of the biggest challenges will be using the various possible combinations in an effective way and designing clinical trials to reflect this, noted de Witte. Sanz stressed the importance of selecting combination regimens that provide efficacy but with limited haematological toxicity. This is important because AZA by itself is myelosuppressive and previous clinical trials of novel agents for MDS have failed due to the phenomenon of additive haematological toxicity. Sanz suggested that triplets may be needed in order to achieve a “cure” in MDS, but that, ultimately, it could prove possible to eliminate HMAs entirely and focus solely on novel agent-based combination regimens.
As the treatment landscape in higher-risk MDS continues to evolve, de Witte asserted his hope that “in the future, allo-HCT may not be necessary anymore.” For now, the best ways to extend and improve results of allo-HCT are to make it less complicated and toxic for the patient (e.g., via reduced intensity conditioning) and to explore the use of immune modulatory therapy post-transplant. Results with HMAs have already highlighted the importance of T cell response, so there is an interesting opportunity to test combination therapies with novel agents in these patients after transplantation using a control arm of no intervention, noted de Witte.
While the future may usher in an era of precision medicine, with specific biomarkers used to drive treatment choices, “currently, we are still at the beginning,” said Sanz. It is, therefore, important to pursue the use of new investigational therapies and novel combination regimens in a range of patients with higher-risk MDS with different underlying somatic mutations. We can take an important step to improve outcomes for patients with higher-risk MDS by taking advantage of an increased understanding of the scientific and biological background of the disease, concluded Santini: combining drugs with different modes of action will pave the way forward.






