Meeting Summary
The main objectives of this symposium were to explore new insights into the biology of multiple myeloma (MM) in the context of new treatment options, discuss the clinical evidence supporting continuous therapy (CT) as a means of enhancing autologous stem cell transplant (ASCT) outcomes, and explore the modern treatment options for patients with relapsed/refractory MM (RRMM), including proteasome inhibitors (PI). Prof Nikhil C. Munshi introduced the latest research on the biology of MM and its possible translation to the clinic and treatment decisions. Prof Pieter Sonneveld then discussed the current clinical knowledge and evidence for the relative roles of ASCT and CT in treating MM in the context of three clinical questions, with expert panel perspectives on each question. Prof Meletios Dimopoulos closed the symposium with an in-depth look at treatment options for RRMM and the results of the TOURMALINE-MM1 trial. Clinical case studies added relevance to these key learnings and demonstrated the importance of a holistic approach to treatment.
New Insight into the Biology of Multiple Myeloma: From Clonal Evolution to New Treatment Options
Professor Nikhil C. Munshi
Prof Munshi opened the presentation with a brief video highlighting the interaction of myeloma cells with the bone microenvironment, the complexities of MM biology and pathophysiology, and the contemporary thoughts on the mechanism of action of proteasome inhibition. Myeloma, similarly to other cancers, begins with a mutation in a single cell and as clonal progression occurs over time new mutations are acquired, leading to monoclonal gammopathy of undetermined significance (MGUS), smouldering MM (SMM), and eventually MM. The terminal MM cell population consists of multiple clones: one predominant clone (the myeloma clone) and a number of minor clones, all of which are competing within the bone marrow (BM) space to gain dominance and the right to survive and evolve. MM undergoes clonal evolution, whereby an ancestral clone acquires an initial driver mutation, either affecting the immunoglobulin heavy chain locus or causing hyperdiploidy, leading to the development of MGUS/SMM. Additional mutations and activation of diverse intracellular pathways such as KRAS and nuclear factor-kappa B (NF-κB) lead to MM. Acquisition of additional mutations can lead to relapse and treatment resistant disease with a poor prognosis.1,2
In addition, interaction with the BM microenvironment can drive further myeloma development.3 Myeloma cells possess a plethora of signalling molecules and express various cell surface molecules (e.g. BAFF-R) that interact with the bone microenvironment and stromal elements to stimulate pathways, such as NF-κB, that are crucial for survival, proliferation, and cell-cycle processes (Figure 1). Interaction with the bone microenvironment also leads to upregulation of cytokines, which, in an autocrine manner, further stimulate myeloma cell proliferation and survival. From a therapeutic perspective, targeting bone microenvironment interactions, stromal cell adhesion, and proteasome activity provides crucial synergy to trigger the death of MM cells.
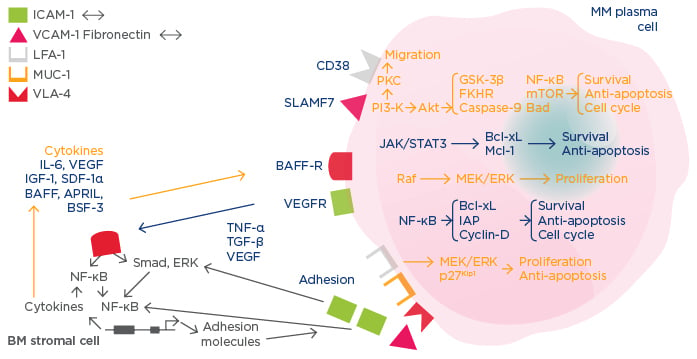
Figure 1: Mechanisms of multiple myeloma disease maintenance and progression.3
MM: multiple myeloma; BM: bone marrow; IL-6: interleukin 6; IGF-1: insulin-like growth factor; VEGF: vascular endothelial growth factor; SDF-1α: stromal cell derived factor-1 alpha; TNF-α: tumour necrosis factor-alpha; TGF-β: transforming growth factor-beta; NF-ΚB: nuclear factor-kappa B; BSF-3: B cell stimulating factor-3; BAFF: B cell activating factor; APRIL: A proliferation-inducing ligand; VEGFR: VEGF receptor; BAFF-R: BAFF receptor; ICAM-1: intercellular adhesion molecule 1; VCAM-1: vascular cell adhesion molecule 1; LFA-1: lymphocyte function-associated antigen 1; MUC-1: mucin 1, cell surface associated; VLA-4: very late antigen 4; mTOR: mechanistic target of rapamycin.
Proteasome Inhibition
Proteasome inhibition affects MM cells in several ways, including disruption of bone microenvironment interactions (e.g. decreased cytokine production), downregulation of cell growth signals and survival, induction of apoptosis through intrinsic and extrinsic pathways, inhibition of the cell cycle, induction of endoplasmic reticulum stress, inhibition of anti-osteoclastic and anti-angiogenic activity, and prevention of heat-shock proteins and DNA repair.3 Myeloma cell survival, regulation of the cell-cycle, and anti-apoptotic mechanisms are all mediated by NF-κB pathways and supported by cytokines and the adhesion of myeloma cells to BM stromal cells. The blockade of proteasomal function reduces secretion of cytokines in myeloma and BM cells and downregulates cIAP2 and Bcl-xL, thus arresting the cell cycle and promoting apoptosis. The proteasome also regulates cell-cycle mediators, such as cyclin D and E, and, when disrupted, the cell cycle is halted (usually in the G2N phase), resulting in decreased proliferation and increased apoptosis. Inhibition of the proteasome also leads to accumulation of misfolded proteins and triggers protein unfolding, both of which cause endoplasmic reticulum stress and ultimately apoptosis.3
Multiple Myeloma Treatment
Currently, there are four main classes of anti-myeloma agents that are commercially available for synergistic use with PIs: histone deacetylase inhibitors, monoclonal antibodies (mAbs), immunomodulatory drugs (IMiDs), and alkylating compounds. Histone deacetylase inhibitors target the alternative pathway of ubiquitinated protein degradation, the aggresome pathway, whereby proteins are degraded by the lysosome rather than the proteasome and work synergistically with PI by blocking a potential mechanism of drug resistance.4 Approved mAbs for combination with PI target CD38 and SLAMF7 to induce apoptosis, complement-dependent cytotoxicity, and antibody-dependent cell-mediated cytotoxicity of MM cells.4 IMiD show various indirect mechanisms of action, including immune mechanisms such as natural-killer cell-mediated killing, suppressor T cell co-stimulation, and pro-inflammatory cytokine downregulation, as well as MM cellular signalling pathways such as cell-cycle arrest, microenvironment effects, and cytokine suppression.5,6
Molecular Biomarkers
Potential biomarkers are being explored to predict which drugs and drug combinations are most likely to be effective in MM. Preliminary evidence indicates that patients treated with PI-IMiD- dexamethasone (Dex) have a greater progression-free survival (PFS) if high levels of c-Myc are expressed (21.4 months) compared with patients with low c-Myc expression (20.6 months; p=0.19), whereas IMiD-Dex displays better activity in patients with low c-Myc expression. This suggests that PI and IMiD may target different clones with different c-Myc levels and differentiation status.7
Proteasome Inhibitors and Treatment Algorithm Development
The central role of PI and combination therapy is highlighted by their use across the MM treatment algorithm. The algorithm focusses on newly diagnosed and relapsed/refractory patients. Newly diagnosed patients can be classified as transplant-eligible or transplant-ineligible based on their age and performance status; those who are transplant-eligible tend to be younger and fitter, and thus generally receive a three-drug regimen, stem cell transplant, and subsequent long maintenance. The latter is sometimes preceded by a short consolidation therapy, then maintenance therapy. Patients who are transplant-ineligible generally receive a two or three-drug regimen, followed by continuous maintenance therapy, especially those at a high risk of early relapse. Patients who have relapsed/refractory disease receive a combination of approved therapies until satisfactory response is achieved; continuous maintenance is then considered (and additionally could be considered for front-line treatment).8,9
Continuous Therapy to Enhance Autologous Stem Cell Transplant: Expert Perspectives
Professor Pieter Sonneveld
Potential benefits of CT include continued cytoreduction and deepening of response, increased likelihood of achieving minimal residual disease (MRD), sustained suppression of the malignant plasma cell clones, prolonged duration of response, improved long-term outcomes, prevention of further evolution of disease, and transforming MM into a chronic condition. However, the potential limitations must also be considered: the feasibility of therapy (e.g. toxicity, cost, quality of life [QoL]), progression on treatment that results in the emergence and selection of refractory myeloma clones, reduced post-relapse survival, and limitation of subsequent therapy choices.10
The aim of CT is to extend remission duration with limited toxic effects. The usual course of patients with active MM shows multiple rounds of remission, active treatment, and relapse, with the quality and duration of each line of therapy becoming reduced with every relapse. The benefit of CT was demonstrated in newly diagnosed transplant-eligible patients in the FIRST trial, where patients treated with continuous lenalidomide-Dex (Rd) were compared with those treated with fixed-duration Rd or with melphalan-prednisolone-thalidomide for 18 months. Those treated with continuous Rd showed a median PFS of 26 months, compared with 21 months for fixed-duration Rd.11 However, CT should only be considered if patients can tolerate the regimen; toxicities should be manageable and not accumulate over time; furthermore, the regimen must be convenient to administer and should have minimal or no impact on patient QoL.
Clinical Question 1: Use of Autologous Stem Cell Transplant or Continuous Therapy in the Era of Novel-Agent-Based Regimens
Prof Sonneveld explored how best to treat transplant-eligible patients in the era of novel agents. The IFM/DFCI trial compared the administration of ASCT after fixed courses of lenalidomide-bortezomib-Dex (RVD) induction with continuous RVD, followed by maintenance in 700 patients with newly diagnosed MM (NDMM). The results showed improved PFS in the ASCT arm (hazard ratio [HR]: 1.5; p<0.0002) and 3-year PFS rates of 61% and 48% in the ASCT and RVD arms, respectively. Complete response (CR) rate was also higher in the ASCT arm (58%) compared with the RVD arm (46%) even though no difference in overall survival (OS) was observed as yet.12 In a similar design, the EMN02 trial compared the administration of bortezomib-cyclophosphamide-Dex as induction followed by ASCT with the triplet combination of bortezomib-melphalan- prednisolone (VMP), both followed by lenalidomide maintenance. Improved PFS was observed in the ASCT arm (HR: 0.73; p=0.01) and deeper responses (represented by very good partial response [VGPR] or higher) were seen in the ASCT arm (84%) compared with the VMP arm (74%).13 In a study by Palumbo et al.,14 patients were randomised to receive either high-dose melphalan plus lenalidomide maintenance, high-dose melphalan plus no maintenance, melphalan-prednisone-lenalidomide (MPR) plus lenalidomide maintenance or MPR plus no maintenance. Both median PFS and 4-year OS were significantly longer in the high-dose melphalan plus ASCT groups (PFS: 43.0 versus 22.4 months p<0.001; OS: 81.6% versus 65.3%; p=0.02, respectively). Maintenance with lenalidomide showed a significant improvement in PFS compared with no maintenance (41.9 versus 21.6 months, respectively; p<0.001), but 3-year OS was not significantly improved (p=0.14).14 In a Phase III open-label study comparing high-dose melphalan plus ASCT with lenalidomide-cyclophosphamide-Dex (CRd), an improved median PFS was observed in the ASCT arm (43.3 versus 28.6 months, respectively). Median 4-year OS was also improved in the ASCT arm compared with CRd (86% versus 73%, respectively; HR: 2.40; p=0.004).15
In conclusion, these studies suggest that the most effective way of treating transplant-eligible patients is with induction therapy plus ASCT followed by maintenance therapy. However, the MRD status at specific time-points should also be considered; MRD negativity has recently been shown to have prognostic implications for substantially improved outcomes, regardless of how it is achieved.16 High rates of MRD negativity can be achieved with novel-agent triplet regimens.17,18 For example, in the EMN02 study, a 79% overall MRD-negative rate was seen (79% in the ASCT arm and 80% in the VMP arm),18 and this led some experts to suggest MRD negativity after induction may remove the need for subsequent ASCT.19 Currently available data are considered preliminary and no randomised studies evaluating outcomes based on MRD status or route of achieving MRD negativity have been published.20
Both the expert panel and the audience agreed that generally patients should receive induction therapy plus ASCT followed by consolidation and/ or maintenance therapy.
Clinical Question 2: Clinical Factors of Importance When Considering Autologous Stem Cell Transplant or Continuous Therapy
Prof Sonneveld explored clinical factors necessitating ASCT or CT. In a Phase III trial comparing melphalan plus ASCT with CRd, an OS benefit was seen in the ASCT arm (HR: 2.40; p=0.004); however, some subgroups did not show a substantial benefit: patients aged ≤60 (HR: 0.89 [95% confidence interval (CI): 0.43, 1.86]) and International Staging System (ISS) Stage 2 and 3 (HR: 1.59 [95% CI: 0.66, 3.62] and HR: 1.42 [95% CI: 0.51, 3.93], respectively), whereas cytogenetic risk had no effect on outcomes (HR: 1.46 and 1.79, respectively, for low and high-risk patients).15 This analysis should be interpreted with caution as it is based on a single trial, and a pooled analysis of several studies showed significant improvements in PFS1 and 2, and OS for ASCT compared with CRd, and an advantage across patient subgroups regardless of prognosis.21
The panel agreed that the benefit of ASCT in high-risk patients is less than in low-risk patients but is still observed; hence, prognostic factors are not considered as determining criteria. However, age must be considered in relation to frailty or to the performance status. Additionally, for patients with high-risk cytogenetics, tandem ASCT may be considered and the ISS status of the patient may guide the subsequent maintenance-therapy choice. The majority of the audience voted that cytogenetics is the most important factor to be considered.
Clinical Question 3: Determining Optimal Post-Autologous Stem Cell Transplant Treatment and the Role of Continuous Therapy
Prof Sonneveld discussed which post-transplant therapies are suitable for long-term continuous treatment. In a meta-analysis of three Phase III studies, CT showed improved PFS1, PFS2, and OS compared with fixed-duration therapy (PFS1: 32 versus 16 months; PFS2: 55 versus 40 months; OS: 69 versus 60 months, respectively) (Figure 2).22 The benefit of maintenance therapy with lenalidomide versus placebo was demonstrated in a meta-analysis of three large Phase III trials; median OS for maintenance was not reached (median follow-up 6.6 years) compared with placebo, 86 months (HR: 0.74 [95% CI: 0.62, 0.89]; p=0.001).23 Subsequently, lenalidomide was approved for maintenance therapy by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA).24,25 With regard to PI, a subanalysis of the HOVON-65/GMMG-HD4 trial that stratified patients by del(17p13) status to receive vincristine-doxorubicin-Dex (VAD) or bortezomib-doxorubicin-Dex (PAD) before or after ASCT demonstrated that patients with del(17p13) benefited the most from PAD treatment (PFS: 26.2 months) compared with VAD (PFS: 12.0 months); p=0.024. This suggests that the adverse impact of del(17p13) on PFS could be significantly reduced by bortezomib-based maintenance.26 The subgroup analysis of the ENM02 trial investigated the role of bortezomib-lenalidomide-Dex consolidation plus lenalidomide maintenance on PFS in patients with low and high-risk cytogenetics, and demonstrated that consolidation treatment benefited the low-risk patients (HR: 0.68; p=0.03) but not high-risk patients (HR: 1.03; p=0.91).
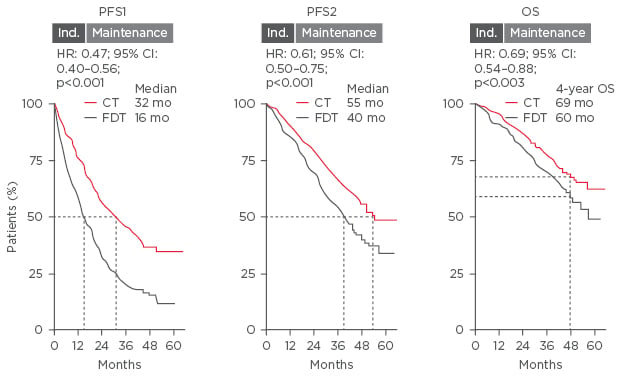
Figure 2: Meta-analysis of three Phase III trials comparing the benefit of continuous therapy with fixed- duration therapy.
CI: confidence interval; CT: continuous therapy; FDT: fixed-duration therapy; HR: hazard ratio; mo: months; OS: overall survival; PFS: progression-free survival.
The panel agreed that, in general, patients receive lenalidomide maintenance indefinitely and most high-risk patients are starting to receive consolidation (usually with lenalidomide-bortezomib) plus maintenance with a PI and lenalidomide. In low-risk patients, consolidation is not default, but may be utilised in certain subgroups. However, Prof Dimopoulos noted that it is not realistic for patients to remain on maintenance for 5–7 years or longer, and that a biomarker such as MRD needs to be validated to allow treatment to be stopped after a certain time.
In conclusion, in the era of novel agents there is still a role for ASCT, with prolonged OS reported with ASCT versus induction therapy alone. There is increasing evidence for the benefit of long-term continuous maintenance therapy, as supported by the recent FDA approval of lenalidomide.24 CT, however, must be tolerable, manageable, without cumulative toxicities, convenient to administer, and should not adversely affect QoL.27
Multiple Options for Relapsed/Refractory Multiple Myeloma: Why Pick Proteasome Inhibition?
Professor Meletios Dimopoulos
Treatment options for patients with RRMM should focus holistically on disease-related factors, treatment-related factors, and patient-related factors, in addition to efficacy. Disease and treatment-related considerations include risk stratification/cytogenetics, renal impairment, prior or existing toxicities, number and type of prior therapies, and history of thromboembolic disease, whereas patient-related factors focus on age/frailty, comorbidities, risk of primary and secondary malignancies, patient lifestyle (e.g. employment and travel preferences), and goals of treatment.28
Key Clinical Considerations
In most studies, high-risk cytogenetics is associated with a poor prognosis.29 In clinical practice it is advisable to check at least for the del(17p) and t(4;14) abnormalities, although t(14;16) and 1q21 may be also relevant in a proportion of patients. The combination of PI and IMiD synergistically reduces the adverse effects of del(17p) and t(4;14) on PFS in NDMM and has received International Myeloma Working Group recommendation.30
Renal impairment is a common comorbidity in patients with MM and, as PI are excreted extra-renally, they are suitable for this patient population;31 however, some cases of acute renal failure have been noted with carfilzomib.32 Lenalidomide and pomalidomide are predominantly excreted through the kidneys and starting doses must be adjusted for patients with moderate-to-severe renal impairment.31
Prior or existing toxicities, such as peripheral neuropathy or poor cardiac function, can limit the use of some PI and should be factored into clinical decision making; for example, in patients with poor cardiac function or uncontrolled coronary disease and/or hypertension who are treated with carfilzomib, overall cardiovascular risk should be closely monitored and controlled. The number and type of prior lines of therapy should be considered when selecting regimens for RRMM, including prior exposure and responses to specific classes of drug, time since prior exposure, and refractoriness to previous regimens.28,33
Age is a factor that is important in the clinical course of MM; after the age of 60 years, the median adjusted time for relative survival decreases from 4.6 years (50–59 years of age) to 3.6 years (60–69 years of age), and OS decreases with increasing age.34 However, age per se is not necessarily the most important variable; frailty, comorbidities, and overall fitness are emerging as more important considerations. The patient’s lifestyle and wishes should be taken into account. Patients may wish to continue work, have an active lifestyle, or may not be able to travel to clinic/hospital regularly, so these aspects would impact on the preferred type of regimen and route of administration. Additionally, inconvenient and time-consuming schedules may affect compliance and feasibility of long-term treatment.
New Treatment Options for Relapsed/Refractory Multiple Myeloma: The TOURMALINE-MM1 Trial
Disease, treatment, and patient-related factors may limit the use of existing parenteral PI; limitations that oral PI may address. The first oral PI to be approved by the FDA and EMA, ixazomib, was approved based on results from the Phase III randomised-controlled TOURMALINE-MM1 trial that compared ixazomib-Rd (IRd) (continuous all-oral PI/IMiD triplet therapy) with placebo-Rd in patients with RRMM.35 The TOURMALINE-MM1 trial randomised 722 patients 1:1 to IRd or placebo-Rd, stratified by prior line of therapy, ISS stage, and previous PI exposure. The primary endpoint was PFS and secondary endpoints included OS, and OS in patients with del(17p). Overall, patient and disease characteristics were well-balanced between arms and patients were representative of the typical myeloma population (median age 66 years; 88% ISS stage 1/2; 19% high-risk cytogenetics). Patients who were proven refractory to lenalidomide or bortezomib were excluded, but patients previously treated with these drugs were allowed to participate, provided they relapsed with a drug-free interval. Patients treated with IRd showed a median PFS of 20.6 months compared with those treated with placebo- Rd (14.7 months), which was a 35% improvement (HR: 0.742 [95% CI: 0.587, 0.939]; p=0.012) (Figure 3). Median time to response and duration of response were 1.1 and 20.5 months, respectively, for IRd, compared with 1.9 and 15.0 months for placebo-Rd. Some patients achieved a CR >1 year into IRd treatment. A greater proportion of patients in the IRd group achieved CR, CR+VGPR, and overall response rate (ORR) compared with the placebo-Rd group (CR: 11.1% versus 6.6%; CR+VGPR: 48.1% versus 39.0%; ORR: 78.3% versus 71.5%).35 Time to progression was significantly longer in IRd (21.4 months) compared with placebo-Rd (15.7 months; p=0.007). Subgroup analysis showed that all patient groups benefited from IRd, including patients who had received multiple lines of therapy.35,36 Prior exposure to PI or IMiD did not appear to impact on IRd treatment (HR: PI-naïve, 0.75 versus PI-exposed, 0.74; IMiD-naïve, 0.70 versus IMiD-exposed, 0.74). Importantly, IRd-treated patients with high-risk cytogenetics showed similar outcomes as low-risk patients (0.54 versus 0.64, respectively), and IRd treatment seemed to entirely overcome the poor prognosis associated with high- risk cytogenetics (low-risk cytogenetics, IRd: 20.6 months, placebo-Rd: 15.6 months; p=0.007; high- risk cytogenetics, IRd: 21.4 months, placebo-Rd: 9.7 months; p=0.021; all PFS).35,36 Patients received a median of 17 and 15 cycles of IRd and placebo-Rd, respectively. The toxicity profile of IRd was similar to the placebo-Rd group; 25% and 20% of patients discontinued due to adverse events (AEs), 74% and 69% of patients experienced any ≥Grade 3 AE, and 47% and 49% of patients experienced a serious AE, respectively. AEs commonly reported with Rd, such as diarrhoea, constipation, nausea, and vomiting, were mostly low-grade and similar in number with the addition of ixazomib. Reports of maculopapular rash, occasionally seen in ixazomib-treated patients, and peripheral neuropathy were similar between treatment arms, and no cardiac, renal, or hepatic signals were noted.35 QoL assessment revealed no additional impact with the addition of a third agent.37 Furthermore, the China Continuation Study showed superior PFS and OS with IRd compared with placebo-Rd.38
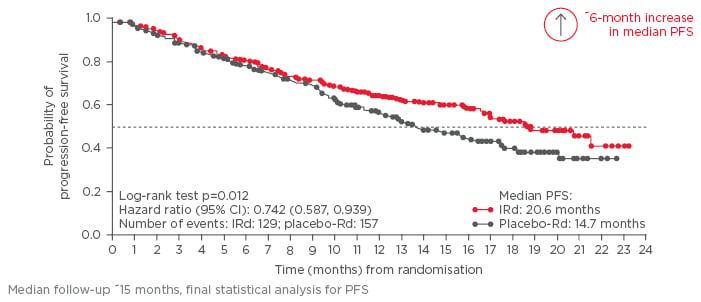
Figure 3: Probability of progression-free survival in the TOURMALINE-MM1 trial.
CI: confidence interval; IRd: ixazomib-lenalidomide-dexamethasone; PFS: progression-free survival; Rd: lenalidomide-dexamethasone.
Ixazomib in the Clinic: Translating Trial Data to Patient Case Studies
The first case study described a 55-year old male who had received three prior lines of therapy (VTD, ASCT, and Rd) but did not have refractory disease, and stopped his treatment due to very good response, with low-risk cytogenetics, ISS Stage 3 disease with mild renal impairment, and limited comorbidities that did not affect activities of daily living. He had full mobility and wished to continue with full-time employment. Factors affecting his treatment selection included disease-related factors of clinical relapse and prior lines of therapy; treatment-related factors of previous exposure, and sensitivity to lenalidomide and bortezomib, and limited duration of control with standard therapies; and patient-related factors of young age, active, in full-time employment, and mild renal impairment. Convenient treatment that does not impact on QoL and conveys low risk of renal complications is thus required. The panel commented that they would consider disease and treatment-related factors the most important for this patient, because he is relatively young and in first relapse; a PI would be recommended.
The second case study presented was a 67-year-old female who had received one prior line of cyclophosphamide-thalidomide-Dex treatment with a limited time to progression, and had del(17p) high-risk cytogenetics, ISS Stage 2 disease with residual Grade 1 peripheral neuropathy. She was retired, cared for by her husband, and led an active lifestyle. Factors affecting treatment selection included the aggressive nature of the disease, limited time to progression on prior therapy, thalidomide refractoriness, residual neuropathy, and partial reliance on a carer; a suitable regimen should have low patient and carer burden. The panel recommended that the patient receive a second induction with a PI/IMiD combination, be considered for ASCT, and maintenance with IRd to control aggressive disease.
The third case study focussed on a 72-year-old male who had relapsed MM following first-line treatment with VMP, low-risk cytogenetics, ISS Stage 2 disease, with cardiac comorbidities and prior myocardial infarction. He had limited mobility and was partially reliant on carers. Important factors for treating this patient included sensitivity to bortezomib, age/frailty, comorbidities, and burden of treatment.
In conclusion, several factors in addition to efficacy should be considered when choosing an appropriate regimen for patients with RRMM. The Phase III TOURMALINE-MM1 study demonstrated the efficacy of IRd in all patient subgroups, regardless of cytogenetic risk, age, prior lines of therapy, and limited additional toxicity versus placebo-Rd. Together with other recently approved drugs, the all-oral IRd combination increases the number of PI-based treatment options available for patients with RRMM.







