Meeting Summary
Three follicular lymphoma (FL) patient case studies were selected by the expert faculty to illustrate the need to individualise treatment and integrate both clinician and patient priorities.
Case one focussed on a 56-year-old female patient with high risk features, for whom the physician sought the treatment that would provide the longest time to next treatment (TTNT) and better efficacy. Treatment was based on the GALLIUM study, demonstrating 4-year progression-free survival (PFS) of 78.1% for obinutuzumab (G) chemotherapy versus 67.2% for rituximab (R) chemotherapy (hazard ratio [HR]: 0.73; 95% confidence interval [CI]: 0.59–0.90; p=0.0034).
Case two was a 72-year-old male patient with comorbidities that limited treatment options. The importance of recognising the impact of comorbidities on treatment choice was considered (the patient’s heart condition made him unsuitable for CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), as well as the practical management of toxicities. The patient, who was treated prior to approval of G, experienced serious Pneumocystis jiroveci pneumonia (PJP) following treatment with R-bendamustine. The need for adequate prophylaxis was emphasised in discussions, with the view that, had the patient been treated in the current era, he may have benefitted from G as long as adequate prophylaxis was started before treatment. There were some discussions regarding the possibility of improving treatment efficacy by changing the antibody and may have reduced toxicity by reducing chemotherapy intensity, for example with G+CVP (cyclophosphamide, vincristine, or prednisone).
Case three referred to a 68-year-old male patient diagnosed with FL and treated first-line with R-CHOP, who concomitantly had a diagnosis of prostate cancer (localised and no need for treatment). Shortly after completing R maintenance, the patient relapsed and refused autologous stem cell transplantation (ASCT) as part of second-line therapy. He was treated with G-bendamustine based on the GADOLIN study (that showed a median PFS of 25.3 months in the G-bendamustine arm versus 14.0 months with bendamustine monotherapy [HR: 0.52; 95% CI: 0.39–9.69; p<0.001] [HR: 0.58; p=0.0061]). The faculty discussed the worse prognosis of early relapsed patients (including patients experiencing disease progression within 24 months of diagnosis [POD24]) and the importance of finding ways to better identify patients at higher risk of early relapse. Some clinicogenetic risk models under investigation, such as m7-FLIPI, were mentioned. The case illustrated the difficulty involved in treating early relapsed patients, and the importance of improving upfront treatment where possible.
Case One: A Young, High-Risk, Newly Diagnosed Patient
Professor Miguel Canales
The first case focussed on a 56-year-old female economist, who presented with abdominal discomfort that led to a CT scan evaluation where lymph node enlargement was found (<3 cm largest diameter). A lymph node biopsy led to diagnosis of FL Grade 1–2 and t(14;18) positive, with a ki67 index of 20%. At diagnosis, bone marrow involvement was detected, with normal complete blood count and blood chemistry (including lactate dehydrogenase [LDH]) and the patient was defined as being Stage IV.
Considering the patient remained relatively asymptomatic, a decision was taken to ‘watch and wait’ for 18 months, after which symptomatic disease progression was documented.
On physical examination, there was an increase in lymph nodes sizes. Additionally, haemoglobin had fallen (Hb 11.8 g/dL), white blood cells increased (21×109/L), and beta-2 microglobulin was elevated (3.1 mg/L). A second biopsy confirmed Grade 1–2 FL (transformation was excluded). PET-CT confirmed disease progression, revealing abdominal bulky disease involving mesenteric, para-aortic, and common iliac lymph nodes (222 mm). At this point, the patient remained a Grade 1-2 FL, Ann Arbor Stage IV, and because this patient was still professionally active, it was considered important to achieve the longest TTNT.
Prof Dreyling discussed the role of FLIPI1 evaluation for treatment decisions: in this case, the patient was graded as FLIPI,2 FLIPI-2,3 and PRIMA-PI high risk.4 In his opinion, FLIPI alone was not necessarily an indication to start treatment and added that he would consider the Groupe d’Etude des Lymphomes Folliculaires (GELF) criteria for decision-making with the tumour bulk being the clear indication for therapy.
Prof Trotman noted that, for this bulky disease presentation, it was surprising that LDH was not elevated and that the patient was asymptomatic. She added that PET-CT scanning alone should not be used to document histologic transformation, noting that standardised uptake value max in abdominal disease was often higher than that of peripheral nodes. In the discussion, the experts emphasised the importance of repeating biopsies at relapse to exclude transformation into high grade lymphomas.
During his presentation, Prof Canales reminded the audience that while the majority of FL patients benefit from R plus chemotherapy, a significant proportion diagnosed with hard-to-treat disease relapse early and even die within a few years of diagnosis. Patients with high-risk FLIPI scores tend to have worse outcomes, irrespective of treatment. Patients with a FLIPI score of 0–1 had a 10-year estimated mortality rate of 4% compared with almost 30% for FLIPI scores of 3–5.2 An analysis of the National LymphoCare study showed FL patients with high FLIPI scores compared with intermediate FLIPI scores experienced lower overall survival (OS), PFS, and TTNT, irrespective of whether the therapy was R-monotherapy, R-CHOP, or R-CVP.3
High-risk patients are difficult to identify, which constitutes an important challenge in the treatment of FL. Prof Canales emphasised the importance of selecting treatment that would prolong TTNT and reduce treatment-related morbidity.
Prof Trotman mentioned data that demonstrate how bendamustine prolongs TTNT. For a younger patient, bendamustine could help prolong TTNT and, most probably at relapse, patients would still be eligible for CHOP if needed. However, with bendamustine there is a trade-off between extending TTNT and increased infection risk. Dr Osborne raised concerns around CHOP (and anthracyclines) in patients with low grade FL. In her opinion, in a patient with bulky disease at presentation, she would recommend investigating the risk of transformation to high grade disease, because this would change clinical management of the patient. If biopsy proved difficult and was clinically low grade, she would prescribe bendamustine as first-line but give antibiotic prophylaxis to cover infection risk. Prof Dreyling added that, for him, because the patient’s LDH was normal it was unlikely that transformation had taken place.
During his presentation, Prof Canales discussed the rationale for the treatment choice for his patient: with currently available treatment options for patients demonstrating survival differences, choosing the best first-line FL treatment can prove challenging. The FOLL05 study comparing R-CVP (n=168) versus R-CHOP (n=165) versus R-fludarabine and mitoxantrone (FM) (n=171) for initial treatment of advanced FL showed R-CHOP delivered a better PFS than R-CVP.5 After a median follow-up of 84 months, the PFS HR for patients treated with R-CHOP compared with R-CVP was 0.73 (p=0.037), which compared to an HR of 0.67 for those treated with R-FM compared to R-CVP (p=0.009).
The StiL study comparing R-bendamustine and R-CHOP found that TTNT was significantly prolonged with R-bendamustine. Median TTNT had not yet been reached in R-bendamustine group while it was 56 months in R-CHOP group (HR: 0.55; 95% CI: 0.41–0.73; p<0.0001).6 In the subgroup analysis, improvement in PFS was not observed for patients with elevated LDH level or high-risk FLIPI score.
FL patients treated with immunochemotherapy experiencing POD24 had poorer subsequent OS than those experiencing progression later (HR: 5.24; 95% CI: 4.63–5.93; p<0.01).7 Furthermore, the study identified male sex, poor performance status (≥2), high FLIPI score (3–5), and elevated baseline beta-2 microglobulin as predictors of disease progression and early death.
The Phase III GALLIUM study compared efficacy and safety of G-chemotherapy (G-chemo) versus R-chemotherapy (R-chemo) in previously untreated patients with advanced stage FL, with chemotherapy backbones (CHOP, CVP, or bendamustine) selected by participating centres. The updated GALLIUM results after 57.3 months follow-up showed a clinically meaningful improvement in investigator-assessed PFS with G-chemo versus R-chemo (HR: 0.73; 95% CI: 0.59–0.90; p=0.0034). The 4-year PFS was 67.2% for R-chemo versus 78.1% for G-chemo (Figure 1).8 Furthermore, a sustained benefit of G-chemo was observed in TTNT (HR: 0.70; 95% CI: 0.54–0.90; p=0.0046).
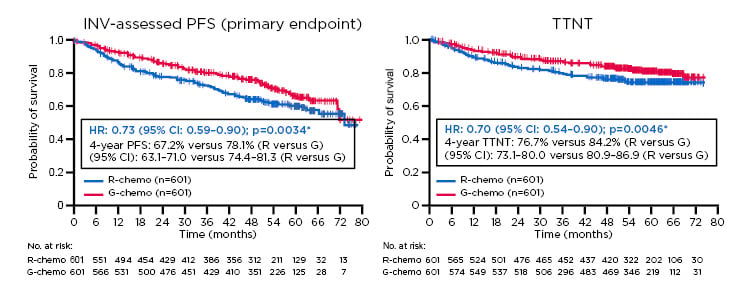
Figure 1: Progression-free survival and time to next therapy results from the Phase III GALLIUM trial.
Previously untreated study participants with advanced stage follicular lymphoma were treated with chemotherapy backbone (CHOP, CVP, or bendamustine) and either G or R. (A) There was a clinical meaningful improvement in investigator-assessed progression-free survival with G-chemo versus R-chemo (HR: 0.73; 95% CI: 0.59–0.90; p=0.0034). (B) Time to next therapy significantly increased with G-chemo versus R-chemo (HR: 0.70; 95% CI: 0.54–0.90; p=0.0046).
ITT populations: *stratified analysis (stratification factors: FLIPI, chemo)
Chemo: chemotherapy; CI: confidence interval; G: obinutuzumab; HR: hazard ratio; INV: investigator; ITT: intention to treat; PFS: progression-free survival; R: rituximab; TTNT: time to next therapy.
Adapted from Townsend et al.8
Further analysis of GALLIUM showed PFS was consistent across chemotherapy backbones, HR 0.63 for G-bendamustine versus R-bendamustine, 0.72 for G-CHOP versus R-CHOP, and 0.79 for G-CVP versus R-CVP.9 The use of different chemotherapy backbones are associated with different side effects, with CHOP associated with higher Grade 3 and 4 neutropenia during the initial phase, and bendamustine associated with higher second malignancies and infection rates during maintenance.9
An analysis of GALLIUM patients in both G-chemo and R-chemo arms found relative mortality at 2 years was 12-fold higher among patients experiencing POD24, as defined earlier (Figure 2).10 Furthermore, fewer early disease progression events occurred with G-chemo (57 out of 601) versus R-chemo (98 out of 601), giving a relative risk reduction of 46.0% (95% CI: 25.0–61.1%).
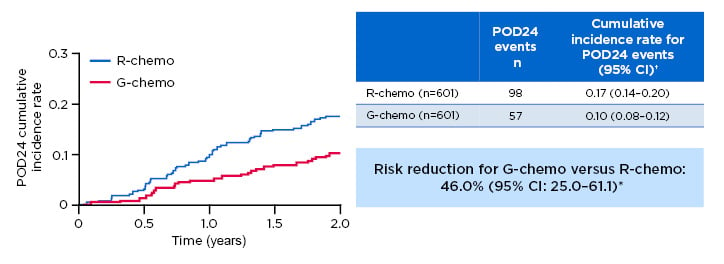
Figure 2: Progression of disease within 24 months of randomisation results from the Phase III GALLIUM trial.
Previously untreated study participants with advanced stage follicular lymphoma were treated with chemotherapy backbone (CHOP, CVP, or bendamustine) and either G or R. There was a lower risk of a POD24 event occurring in those who received G versus R (57/601 versus 98/601, respectively; relative risk reduction: 46.0%; 95% CI: 25.0–61.1%).
*Risk reduction based on (1-HR) x 100; †2-year cumulative incidence rate.
Chemo: chemotherapy; CI: confidence interval; HR: hazard ratio; FL: follicular lymphoma; G: obinutuzumab; POD24: progression of disease within 24 months of randomisation; R: rituximab.
Adapted from Seymour et al.10
Prof Canales gave this patient G-CHOP; during his presentation he explained the reason for choosing G-CHOP to treat this first-line FL patient:
- Firstly, the SWOG S0016 trial demonstrating a 10-year PFS for R-CHOP of 42%.11
- Secondly, the PRIMA study showing combining R-chemo + R-maintenance resulted in a 10-year PFS of 51%.12
- Lastly, the GALLIUM trial providing evidence that G-CHOP is superior to R-CHOP and reduces progression risk overall by 28%, with an additional benefit of risk of disease progression within 24 months by 46%.9 Prof Canales felt that G-based immunochemotherapy may provide several more progression-free years over R-chemo.
In this case, the patient also received prophylaxis for PJP.
Observed adverse events were also discussed, namely one Grade 1 infusion related reaction and neutropenia (Grade 3) during the first cycle. For subsequent cycles, the patient received secondary prophylaxis with granulocyte colony-stimulating factor and completed a full dose of chemotherapy without additional problems.
Response evaluation at the end of induction with PET-CT showed the patient to be in complete remission. Following the six cycles of G-CHOP induction, the patient was prescribed G-maintenance for 2 years as recommended in the Summary of Product Characteristics, and with ongoing G-maintenance the patient remained in complete response. She was back at work feeling well at the time this symposium took place.
While G has convincing data for use in first-line FL, especially in younger high-risk patients, a drawback is the 4-hour infusion time. Prof Canales mentioned the Phase IV GAZELLE study, a clinical trial currently investigating faster G-infusions over 90 minutes in previously untreated FL patients.13
The case, which considered a young high-risk FL patient needing systemic treatment, demonstrated that the treatment option choice should take into consideration the possibility of achieving a more efficacious response and longer TTNT. In this case, G-CHOP was chosen.
CLICK TO VIEW THE VIDEO Prof Canales’ Presentation
Case Two: An Older Intermediate-Risk Patient with Comorbidities
Doctor Wendy Osborne
Dr Osborne presented the case of a 72-year-old male with comorbidities including metabolic syndrome and myocardial infarction in 2005. The patient is a self-employed taxi driver who provides the only income for his family, so time off work has a major impact for him. In 2011, after presenting with axillary lymphadenopathy, a CT scan and axillary node biopsy revealed FL Grade 1–2.
The patient was diagnosed with asymptomatic Stage 3 disease and put on ‘watch and wait’. Currently, in the UK, four doses of single-dose rituximab can be offered to patients with advanced asymptomatic FL as it was considered to be of health economic benefit by National Institute for Health and Care Excellence (NICE), a decision that was mainly taken based on health economics due to increasing TTNT.14 In practice, this is rarely done and most patients are managed on a watch and wait approach.
The patient experienced a second myocardial infarction in May 2012, necessitating three drug-eluting stents. He stayed on surveillance until December 2015 when he developed left groin adenopathy. Dr Osborne stressed the alignment of patient and physician goals. For this patient, it was important to minimise time off work, making it more relevant to maximise TTNT. Dr Osborne’s treatment goals were to maximise TTNT, minimise toxicity (especially cardiovascular), and reduce infection risk.
During her presentation, Dr Osborne also argued the reason for the choice of chemotherapy for this patient. As a result of StiL study results, demonstrating a PFS survival advantage for R-bendamustine over R-CHOP (HR: 0.61; 95% CI: 0.42–0.87; p=0.0072).15 Dr Osborne has since moved many of her patients (including the case study) to bendamustine if they were able to tolerate it. Had it been approved when the patient was treated, Dr Osborne would have selected G to try for the longest TTNT, by changing the anti-CD20.
In the panel discussion about bendamustine, Prof Trotman discussed with the faculty her reluctance to prescribe bendamustine in patients >70 years old with diabetes, because this comorbidity increased the risk of infection. She added that a sub-analysis of the GALLIUM study that considered patients according to chemotherapy partner had shown a mortality rate of 13% in patients >70 years of age, regardless of whether they received R with G or bendamustine.9
Prof Dreyling felt that an important question was how much bendamustine should be used, i.e., the preferred number of cycles and dose. In retrospect, Dr Osborne agreed bendamustine may not have been the best choice for the patient. The rationale behind the stronger treatment was to treat the patient once to achieve functional cure and avoid further relapses due to concerns he would not be able to tolerate chemotherapy.
Following up on these topics, the faculty addressed that infections represent a major concern; GALLIUM revealed 5% mortality from infections demonstrating the need for prophylaxis. There was discussion regarding the different dosing regimens for bendamustine. Prof Trotman raised the concern that there are no supporting data for a reduction in the number of cycles of bendamustine for the elderly, as much as one might endorse the approach intuitively, and consideration needs to be made of a response-adapted approach in patients with delayed neutrophil recovery and/or infections in their later cycles. The panel discussion highlighted the need to personalise chemotherapy backbones to patients. The patient’s heart condition made him unsuitable for CHOP, making CVP possibly a better option; however, even with CVP, the patient would be likely to progress or relapse within 18 months to 2 years.
Dr Osborne continued the case presentation, informing the audience that she always screens for hepatitis B, C, and HIV. A high proportion of FL patients with previous hepatitis B infections will have a reactivation of the infection when prescribed chemotherapy,16 emphasising the need for screening and the involvement of the liver team before chemoimmunotherapy starts and during the course of induction and maintenance. The patient, who was hepatitis B core antibody-positive and surface antigen-negative, commenced lamivudine prophylaxis, continuing antiviral prophylaxis throughout treatment. In this case, the patient started R-maintenance that had to be stopped after 5 months because of neutropenia and two admissions for neutropenic sepsis.
Dr Osborne mentioned that the 10-year follow-up PRIMA data showed a PFS benefit was achieved but there was no OS benefit. Dr Osborne mentioned that while there are several studies currently exploring the effectiveness of maintenance therapy, her current approach is to offer maintenance therapy to her patients based on the increase in PFS. PRIMA results for FL patients after response to a first-line R-chemotherapy induction showed PFS at 3 years was 75% for patients receiving R-maintenance versus 58% for patients under observation (HR: 0.55; 95% CI: 0.44–0.68; p<0.001).12 However, OS at 10 years was not significantly different: 80% for R-maintenance versus 80% for observation (HR: 1.04; 95% CI: 0.77–1.40; log-rank p=0.795).
Prof Trotman commented on this case, and while she would have started maintenance in this patient, she would have stopped at the first infection. Prof Canales agreed with the decision to start maintenance but added that patients often experienced anaemia in addition to neutropenia resulting in infectious complications, making it necessary to initiate prophylaxis with first-line bendamustine. Prof Dreyling added that when discussing maintenance PFS benefits with patients, he found around 90% of them chose maintenance. As a conclusion, the panel addressed the importance of evaluating each patient’s infectious risk profile and being careful in deciding on early prophylaxis and treatment adjustment according to observed toxicities.
In March 2019, this patient was admitted with dyspnoea and nearly died from PJP pulmonary infection, as he had not received PJP prophylaxis.
Dr Osborne discussed the GALLIUM data that demonstrated how fatal events are more frequent among patients treated with bendamustine combinations, regardless of the anti-CD20 used.9,17 Data from this study showed mortality was 4.7% for patients receiving R-bendamustine and 5.9% for patients receiving G-bendamustine, contrasting with 2.0% for R-CHOP, 1.6% for G-CHOP, 1.8% for R-CVP, and 1.6% for G-CVP. According to Dr Osborne, mortality occurred in the maintenance period as a direct result of T-cell depletion secondary to bendamustine. Realisation of the link between bendamustine and mortality was practice-changing for Dr Osborne, resulting in her giving her patients PJP prophylaxis, regardless of whether they have maintenance or not. Dr Osborne saw this patient several weeks before this symposium and he was doing well. The fact that the patient was treated with R, as opposed to G, supports the concept that high-risk opportunistic infections such as PJP may not be related to the choice of anti-CD20 antibody.
In summary, this case shows once more the importance of individualising FL treatment decisions, as well as the importance of aligning physician-patient treatment goals and involving patients in making treatment decisions for both the anti-CD20 and chemotherapy partner. The discussion highlighted the importance of screening for hepatitis, having a low threshold to stop maintenance, and using PJP prophylaxis with bendamustine.
CLICK TO VIEW THE VIDEO Dr Osborne’s Presentation
Case Three: A High-Risk Patient Failing First-Line Therapy
Professor Martin Dreyling
The third case referred to a 68-year-old Bavarian man who presented with gastrointestinal symptoms in January 2012. The investigation led to the diagnosis of an 8 cm abdominal bulk mass and a biopsy confirmed FL (Grade 2) Stage IIIb, FLIPI-2. Around the same time, the patient was diagnosed with prostate cancer (Stage pT1c, Grade 2, Gleason 6), and a watch and wait approach was adopted.
The patient was treated with R-CHOP induction followed by R-maintenance, but <6 months after maintenance the patient developed symptoms and a progressive abdominal bulk was diagnosed (Grade 1). As the patient did not attend many of his follow-up appointments it was unclear exactly when the patient relapsed, but most likely before Month 27.Dr Osborne commented on the POD24 data first generated by Dr Carla Casulo in R-CHOP treated patients,18 and that less is known about POD24 in the context of bendamustine treatment. However, with the current patient she agreed prognosis was likely to be <5 years. Prof Canales was in agreement, adding that the therapeutic options were limited in clinical practice for such patients who had a poor prognosis. Prof Trotman recommended radiological review from a multidisciplinary team to decide if the patient had minor increase in lymph nodes only, or a notable rapid progression. Prof Dreyling added that it was unimportant whether progression occurred at 18, 24, or 30 months; it was of greater importance to differentiate between early and late relapse in general. In this discussion, Prof Dreyling addressed the POD24 utility to predict early treatment, having been validated by two independent cohorts of symptomatic FL patients undergoing first-line immunochemotherapy (a German Low-Grade Lymphoma Study Group [GLSG] trial in which POD24 occurred in 17% of patients and the British Columbia Cancer Agency [BCCA] registry in which it occurred in 23%). Results showed 5-year OS rates in GLSG were 41% for POD24 versus 91% for those without POD24 (p<0.0001), and in BCCA were 26% for POD24 versus 86% without POD24 (p<0.0001).19
Prof Dreyling focussed part of his discussion on the need to improve risk stratification to help selection of first-line FL immunochemotherapy.
This has been addressed by development of a clinicogenetic risk model called m7-FLIPI, incorporating both FLIPI and ECOG status, as well as the mutational status of seven genes (EZH2, ARID1A, MEF2B, EP300, FOX01, CREBBP, and CARD11).20 FLIPI alone showed that 51% of patients were predicted to be high-risk, but when mutation profiling was added, this high-risk group was split, with 28% predicted to be very high-risk. A French team used gene expression profiling data to build a predictive outcome model with 23 genes associated with progression.21 In two separate studies, the team showed that the model revealed statistically significant differences in achieving PFS between those patients achieving high scores on the model and those achieving low scores. In both training and validation cohorts the team showed statistically significant differences between probabilities of achieving PFS between low-risk predictor and high-risk predictor scores (p<0.001). In the future, a simplified score is likely to be developed, including gene mutations and expression of genes.
Regarding optimal treatment for first relapse in R-refractory FL, Prof Canales and Dr Osborne commented on their selection of G-bendamustine, and Dr Osborne added that if patients were fit, she might offer ASCT. Prof Trotman felt there was balance between the benefits achieved by ASCT and G-bendamustine.
Prof Dreyling also mentioned the current European Society for Medical Oncology (ESMO) guidelines, recommending that in early FL relapse, Stage III/IV disease, high-dose consolidation with ASCT should be discussed with patients.22 Several studies have addressed the benefits of ASCT in relapsed/refractory FL patients (such as those in GLSG1996 and GLSG2000 trials) and have demonstrated survival benefits associated with ASCT. The 5-year second-line PFS for ASCT versus no transplant was 51% versus 19% (HR: 0.38; p<0.0001), and a 5-year second-line OS of 77% for ASCT versus 59% for no transplant (HR: 0.54; p=0.031). Despite the evidence, the patient refused ASCT.
Considering the context outside of ASCT, Prof Dreyling discussed the results of studies in relapsed FL, namely:
- The GADOLIN trial, in which patients with R-refractory indolent non-Hodgkin lymphoma were randomised 1:1 to induction with G-bendamustine + G-maintenance (n=164) or bendamustine monotherapy (n=171). Results showed median PFS was 25.3 months in the G-bendamustine arm versus 14.0 months with bendamustine monotherapy (HR: 0.52; 95% CI: 0.39–0.69; p<0.001) (Figure 3).23 OS was also prolonged, with median OS not reached for G-bendamustine versus 53.9 months for bendamustine (HR: 0.58; 95% CI: 0.39–0.86; p=0.0061).
- As an alternative for this patient, it was considered whether lenalidomide+R was suitable. Prof Dreyling mentioned that, following a presentation at the American Society of Hematology (ASH) 2018, data has been submitted to the U.S. Food and Drug Administration (FDA) for approval. However, in an ideal world, in his opinion, the combination of lenalidomide and G would be given. The side effects of lenalidomide, such as fatigue, should not be underestimated. The S1608 trial (currently ongoing) is randomising patients with early progression or refractory FL to G-lenalidomide, G-CHOP, or TGR-12012+G.24
Prof Dreyling revisited the delayed toxicity of bendamustine in a study of 2 versus 4 years of R-maintenance following bendamustine+R induction. In this study, CD4+ cell counts rise slowly after the end of induction and take years to recover completely.25
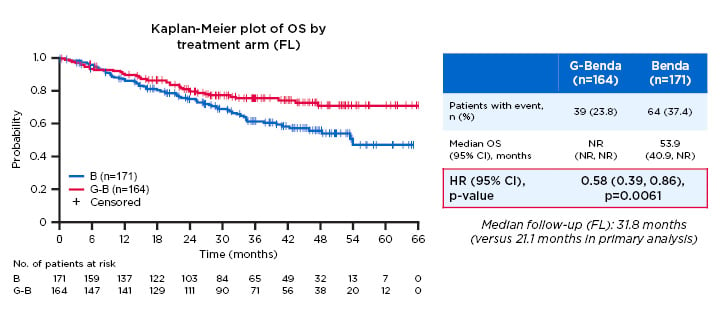
Figure 3: Overall survival in the follicular lymphoma population of the GADOLIN study.
Study participants with R-refractory indolent non-Hodgkin lymphoma were randomised 1:1 to induction with G-bendamustine + G-maintenance (n=164) or bendamustine monotherapy (n=171). A significant improvement in overall survival duration was observed in the G-bendamustine group versus the bendamustine monotherapy group (median overall survival not reached versus 53.9 months, respectively; HR: 0.58; 95% CI: 0.39–0.86; p=0.0061).
B: bendamustine; benda: bendamustine; CI: confidence interval; G: obinutuzumab; HR: hazard ratio; NR: not reached; OS: overall survival.
Adapted from Cheson.23
In the GALLIUM study, it was demonstrated that Grade 3/4 neutropenia occurs less frequently during induction in patients treated with bendamustine than those treated with CHOP or CVP.
At relapse, the patient was treated with G-bendamustine according to the GADOLIN trial and received six cycles of G-bendamustine as induction, followed by G-maintenance for 2 years (obtaining a partial response). Unfortunately, this patient relapsed 1 year after G-maintenance and initiated a third-line treatment with the P13K inhibitor, idelalisib, a therapy targeting multiple signalling pathways (including P13K delta), that has been shown to have a high response in double refractory FL.26
This particular patient demonstrated the heterogeneous clinical course that relapsed FL can take, with the major prognostic factor being prior duration of remission. Optimal salvage treatment improves OS, with younger patients experiencing early relapse benefiting from ASCT and R refractory and elderly patients benefitting from G-bendamustine. In this case, Prof Dreyling mentioned that the patient experienced colitis, a class-specific effect adverse event of PI3K delta inhibition,27 demonstrating that chemotherapy-free options do not necessarily mean no toxicity and, furthermore, the need to individualise treatment for each patient.
CLICK TO VIEW THE VIDEO Prof Dreyling’s Presentation
What we have Shared about Managing Follicular Lymphoma
Professor Judith Trotman
Prof Trotman ended the symposium by summarising the discussion. The three case studies demonstrate how patient outcomes are continually improving towards a functional cure. Although risk stratification is under development, no comprehensive, readily available tools exist for identifying all high-risk patients. Treatment choices are dependent on multiple factors including conventional clinical risk factors, along with patient comorbidities, and individual preferences. Clinicians need to be well informed of the relative merits and toxicities of the comprehensive array of chemoimmunotherapy options now available. Such knowledge enables clinicians to guide each patient in choosing the ‘right treatment at the right time’.







