Meeting Summary
The meeting was arranged as a series of conversations between experts, following a question and answer format with two speakers in each presentation. In the first presentation, Dr Soverini and Prof Lion discussed the importance of the timing and depth of response with respect to clinical outcomes in Philadelphia chromosome positive (Ph+) leukaemias. They showed how sensitive and reproducible measurements of molecular response (MR) and the proper interpretation of laboratory data are critical to correctly inform therapeutic decisions in patients with chronic myeloid leukaemia (CML) and Ph+ acute lymphoblastic leukaemias (ALL). Detection of BCR-ABL mutations can establish the need for treatment change and, in some cases, indicate which tyrosine-kinase inhibitor (TKI) is most likely to be effective. The speakers addressed the need for more sensitive and accurate methods to monitor minimal residual disease (MRD) and detect mutations that drive resistance to TKI therapy. They explored two distinct patterns of mutation observed in patients with >1 mutation (polyclonal and compound mutations) and how in addition to selecting the most appropriate TKI it is also important to consider the most appropriate dose.
In the second presentation, Dr Bassan and Prof Dr Junghanß discussed the evolving treatment landscape for Ph+ ALL, including the role of TKI, chemotherapy, and allogenic stem cell transplantation (SCT). The advent of TKI has improved the prognosis for Ph+ ALL, allowing many more patients to achieve complete remission and be considered for allogeneic SCT. However, treatment-related mortality remains a significant issue after allogenic SCT affecting 20–33% of patients.
Studies show that early death rates are lower for patients receiving ‘light’ chemotherapy and TKI with steroids in place of chemotherapy. Furthermore, for patients achieving complete MR, in some studies there is no difference in outcome between those who undergo allogenic SCT and those who do not, provided that the latter subgroup was selected according to absence of residual disease by PCR analysis. Such data suggest that, in Ph+ ALL, novel therapeutic approaches may in some patients obviate the need for intensive chemotherapy and allogeneic SCT. Studies are now ongoing to explore whether Ph+ ALL patients can abstain from allogenic SCT through selection of the strongest TKI upfront and whether chemotherapy-free regimens might be an option.
Screening and Monitoring Approaches to Optimise Treatment in Philadelphia Chromosome Positive Leukaemias
It is well established in CML that achieving MR milestones at defined time points controls the trajectory towards optimal response and treatment free remission.1
The concept of early MR (EMR) is prognostically important, but, in recent years, the kinetics of BCR-ABL transcripts in the first 3 months have been shown to be a more reliable and accurate indicator of disease state than a single measurement at 3 months. An Australian study2 demonstrated that BCR-ABL value from baseline halving time of <76 days in patients on first-line imatinib predicted progression-free survival and overall survival. Similarly, a German study showed that reduction of BCR-ABL transcripts of half a log or more within the first 3 months is also associated with better survival outcomes.3-5
Although studies suggest EMR kinetics offer prognostic information, this has not yet been incorporated into the recommendations published by European LeukemiaNet (ELN) or the National Comprehensive Cancer Network (NCCN), because precise cut-off levels have not yet been defined and technical issues (e.g., the need for a different control gene) still hamper prompt application of this approach.
The time taken to achieve MR is important, with CML patients that achieve major MR at 3 months having a higher cumulative incidence of achieving MR4.5 after 8 years of receiving imatinib than those achieving major MR at 6, 12, or 18 months.6 Patients demonstrating good imatinib responses can take 5 years or more to reach MR4.5, the deep MR required for treatment free remission in most studies. Second generation TKI can achieve these levels of deep MR much more rapidly.4
Provisional criteria for selecting the best candidates for TKI discontinuation proposed by the NCCN include stable MR (MR4.0; BCR-ABL ≤0.01% IS) for ≥2 years documented on at least 4 tests performed at least 3 months apart.7 Results for a range of TKI in CML show that relapse-free survival with at least major molecular remission was achieved for 33–68% of patients after 0.5–7.0 years of treatment.8
Detection and monitoring of MR requires reliable diagnostics. The importance of selecting reliable laboratories is underlined by a EUTOS laboratories review showing that around 17% were unable to reliably score MR4.5 levels in 2017 (unpublished data).
Monitoring of BCR-ABL transcript levels in Ph+ ALL is also considered valuable. Monitoring MRD helps determine who should receive allogeneic SCT and who should receive post-transplant therapies. Furthermore, MRD positivity predicts haematological relapse after allogeneic SCT, even with TKI therapy.9
Monitoring of MR with real-time quantitative PCR of BCR-ABL levels is the method of choice for Ph+ ALL management. Precise cut-offs for MR levels have not yet been defined,10 although the European Working Group for Adult ALL (EWALL) and the European Study Group-MRD-ALL consortium are looking to standardise methodologies, reduce variability, and optimise procedures.
The most common currently known mechanism behind TKI resistance is BCR-ABL mutations.11 ELN and NCCN guidelines recommend BCR-ABL kinase domain mutation screening should be undertaken in chronic phase CML patients failing to reach response milestones, at any sign of loss of response, on disease progression, and for patients presenting in accelerated or blast phase.12 The ELN currently recommends Sanger sequencing to detect BCR-ABL mutations, although a EUTOS study13 showed Sanger sequencing only detects clones representing 20% of the entire leukaemic load.
Two distinct patterns have been observed for patients with >1 mutation: polyclonal mutations (mutations existing separately in different clones) and compound mutations (different mutations found in the same BCR-ABL protein)14 (Figure 1). Data show compound mutations are harboured by 3% of chronic phase CML patients, 30% of accelerated phase or blast phase CML patients, and 35% with Ph+ ALL who are positive for mutations (Soverini S, personal communications).
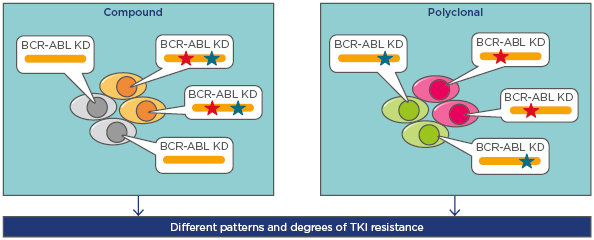
Figure 1: The presence of more than one BCR-ABL mutation in Philadelphia positive leukaemias: compound and polyclonal mutations.
BCR-ABL compound mutants present with two mutations within the same BCR-ABL molecule, whereas the mutations are in separate clones in the case of polyclonal mutations. Mutations are shown by the red and green stars.
KD: kinase domains; TKI: tyrosine kinase inhibitors.
Adapted from Khorashad et al.14
Published methodologies for detecting polyclonal and compound mutations include next-generation sequencing (NGS) of short overlapping fragments (where four overlapping fragments were used to cover the kinase domain),15 long range NGS (allowing the kinase domain to be covered in a single read; not commercially available any more),16 and NGS on the PacBio® platform (Pacific Biosciences, Menlo Park, California, USA), allowing reading of longer stretches.17 TK domain mutations detected by NGS sequencing allow a more accurate picture of BCR-ABL mutation status, enabling better TKI selection and mutations to be detected earlier than by Sanger sequencing.15,18-21
An Australian study22 demonstrated that the number of low burden mutations was inversely associated with failure free survival. This finding was supported by a study showing patients without any mutations as assessed by NGS had significantly longer progression-free survival compared to those with mutations (p=0.041).23 Such data suggests patients with complete cytogenetic response (but not with major MR) should be screened regularly for mutations.
In Ph+ ALL, the complexity of mutant subclones has been shown to be higher than in CML, with genetic instability of the BCR-ABL gene in Ph+ ALL leading to early accumulation of point mutations.24 At diagnosis, low burden mutations are detected in >20% of Ph+ ALL patients.25-28
The swift emergence of mutations and greater genetic complexity of Ph+ ALL poses clinical and diagnostic challenges with NGS sequencing of BCR-ABL mutations providing the basis for improved outcomes. Screening can identify different mutant subclones, the percentage of affected cells, and presence of compound mutations. Digital PCR permits absolute quantification of BCR-ABL molecules and facilitates assessment of the size of specific mutant subclones, but the currently available spectrum of mutations detectable on this platform is limited.
NCCN guidelines provide treatment recommendations for 12 of the most commonly occurring BCR-ABL1 mutations.7 In the absence of guidelines, use of IC50 heat maps to select second-line TKI should be viewed with caution because different heat maps produce conflicting results.29,30 TKI doses can also have a major influence, with doses achieving higher plasma concentrations being more likely to be effective against certain mutations.30-32
Compound mutations were thought to be broadly resistant to TKI, but a recent study suggests that there are three categories of compound mutations and the efficacy of ponatinib is different for each.33
- Those with an IC50 considered achievable even with the lowest dose of ponatinib.
- Those with very high IC50 values not achievable with any clinically feasible dose of ponatinib (particularly compound mutations including T315I or F317L).
- Those with IC50 values not achievable with lower doses but achievable with higher doses of ponatinib.
Such data underline that, in addition to selecting the most appropriate TKI, it may also be important to consider appropriate dosing regimens.
Evolving Strategies for The Management of Philadelphia Chromosome Positive with Acute Lymphoblastic Leukaemia
In the era of TKI, clinical trials demonstrate significant improvements in Ph+ ALL survival. Studies show adding any TKI to chemotherapy gives a 5-year survival rate of 35–50%, compared to pre-TKI studies showing survival rates around 20%.34-38
Before the introduction of TKI, patients with Ph+ ALL disease had much worse outcomes than those with Ph negative (-) ALL. A population based study (using USA Surveillance, Epidemiology, and End Results [SEER] data in the TKI era) showed no survival difference between Ph+ ALL and Ph- ALL, in the 18–39 year age group (p=0.46); however, older patients (>40 years) with Ph+ ALL had a slight but significant survival advantage over Ph- ALL patients (p=0.037).39 Explanations for improvements in Ph+ ALL survival include addition of TKI to standard treatment increasing rates of complete remission, allowing allogeneic SCT to take place.40
Data from the European Society for Blood and Marrow Transplantation (EBMT) showed ALL accounted for 16% of allogenic SCT in 2015, and that ALL transplants across Europe increased from around 1,000 per year in 1998 to 2,500 per year in 2014. The rise can be attributed to marked increases in unrelated donors (due to tissue typing improvements), which increased donor availability (Figure 2).41
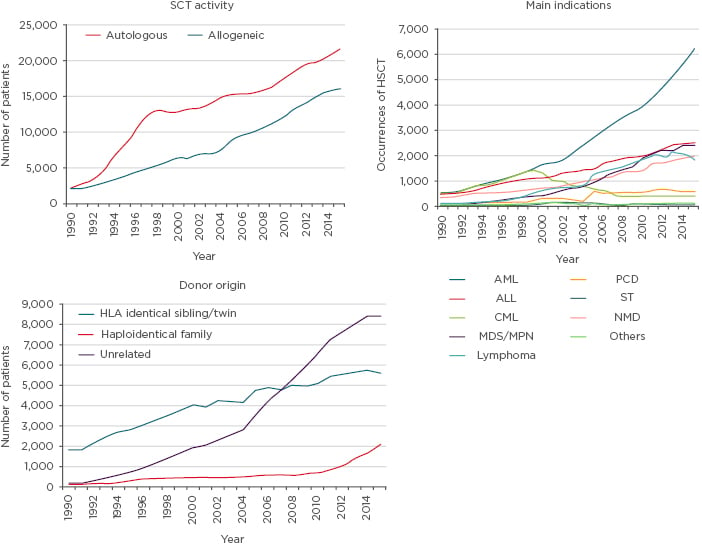
Figure 2: Data from the European Group for Blood and Marrow Transplantation Activity Survey 2015 showing information on stem cell transplantation.
ALL: acute lymphoblastic leukaemia; AML: acute myeloid leukaemia; CML: chronic myeloid leukaemia; HSCT: haematopoietic stem cell transplant; MDS: myelodysplastic syndrome; MPN: myeloproliferative neoplasms; NMD: non-malignant disorder; PCD: plasma cell disorder; SCT: stem cell transplantation; ST: solid tumour.
Adapted from Baldomero and Passweg.41
To allow cure by allogeneic SCT it is important to achieve complete remission with TKI prior to transplant. Regarding TKI availability, currently only imatinib is licensed in Europe for frontline ALL treatment, with dasatinib allowed in imatinib resistant or intolerant patients and ponatinib licensed after dasatinib failure or for patients with T315I mutations. Nilotinib, bosutinib, and ponatinib are available through clinical trials.
Important concepts for allogenic SCT include obtaining complete remission rates close to 100% and MRD remission (<10-4), avoiding loss of response, MRD positivity, and therapy related mortality. Few relapsed/refractory ALL patients survive for >1 year, even with new therapies such as ponatinib, inotuzumab ozogamicin, and blinatumomab.42-44
Clinical trials show TKI (imatinib, nilotinib, dasatinib, or ponatinib) achieve similar complete remission in ALL (90% plus).45 However, higher rates of complete MR can be achieved for nilotinib (MR5: 86%)46 and ponatinib (MR5: 78%)47-48 compared to dasatinib (MR5: 24%).27,49,50
Studies demonstrate that achieving complete MR prior to allogenic SCT reduces risk of relapse. A representative study analysing Ph+ ALL transplant patients receiving imatinib showed cumulative incidence of relapse was 86.1% for patients achieving poor MR compared to 5.1% for EMR, 6.1% for late MR, and 16.9% for intermediate MR.51 Clinicians need to take comorbidities into consideration, making use of the Haematopoietic Cell Transplantation Specific Comorbidity Index to score factors such as cardiac conditions, inflammatory bowel disease, diabetes, and obesity.52 The index can be used to discuss transplant related mortality risks. Studies have shown treatment related mortality after allogenic-SCT affects 20–33% of patients,53,54 creating the challenge of identifying the patients for whom transplant should be omitted.
TKI can be used to prevent relapse after allogeneic SCT. In the only randomised study using imatinib following allogeneic SCT in Ph+ ALL, there was no difference in 5-year survival (p=0.84) or event free survival (p=0.89) between SCT patients receiving prophylactic imatinib or treatment following detection of MRD.55 However, the study showed molecular relapse was 69% in the MRD triggered approach group versus 40% in the prophylactic group. Both approaches have advantages and disadvantages, with the MRD triggered approach involving less toxicity and the prophylactic approach less molecular relapse.
In 2016, the EBMT’s Acute Leukemia Working Party published a position statement on TKI use according to pre and post-transplant MRD status.56 Recommendations included:
- Patients who are MRD and preallogenic SCT that had become negative should receive prophylactic TKI according to pre-transplant mutation status or observation and TKI when MRD positive.
- That patients who are MRD positive, preallogenic SCT, and remain MRD positive (or MRD negative preallogenic SCT and become positive) should be checked for BCR-ABL kinase domain mutations and receive TKI according to mutation status.
- Patients who are MRD negative before transplantation and remain MRD negative after transplantation should receive prophylactic imatinib or be observed to screen for becoming MRD positive and receive TKI according to mutation status.
A clear message from trials exploring chemotherapy combinations is that light chemotherapy regimens avoid induction mortality. Studies show early death rates for patients receiving intensive chemotherapy in combination with TKI are 4.0–8.8%;34,46,57 for non-intensive chemotherapy plus TKI, these rates are 0.0–4.2%; 27,34,54 and for TKI (with steroids) without chemotherapy they are 0.0%.50,58,59
Recent studies using nilotinib or ponatinib in newly diagnosed Ph+ ALL showed no difference in overall survival between patients receiving and not receiving chemotherapy, raising the possibility of patients avoiding chemotherapy.47,46,59-61 Furthermore, a poster60 was presented at the European Hematology Association (EHA) 2018 Congress that found no difference in outcomes for Ph+ ALL patients with complete MR who underwent transplant and those who did not undergo transplantation. Such data suggest allogenic SCT might be avoidable in patients with complete and durable MR.
A number of trials are currently exploring whether Ph+ ALL patients can abstain from allogenic SCT through use of dual therapies, including the MDACC trial and the D-ALBA Frontline Sequential Dasatinib and Blinatumomab in Adult Philadelphia Positive Acute Lymphoblastic Leukemia study, combining second or third-generation TKI with a monoclonal antibody alone or with corticosteroids, respectively. Additionally, the EWALL03 study in Ph+ ALL patients above the age of 55 years with no transplant options (due to age) will be comparing deintensified chemotherapy plus first and third-generation TKI. Furthermore, in frontline Ph+ ALL, a Takeda study across all age groups is comparing different TKI regimens. In conclusion, TKI have been shown to confer clear benefits on Ph+ ALL patients, with new possibilities to reduce risk of mortality by reducing chemotherapy and allogenic SCT.
Click here to watch an interview with Dr Soverini.







