Meeting Summary
Prof Bruce Cheson opened the symposium by highlighting the unmet needs for patients with follicular lymphoma (FL) and the potential application of prognostic scores, imaging techniques, and genomics to stratify patients. Ms Rosmarie Pfau detailed the challenges faced by patients with FL around the world, particularly a desire for improved quality of life (QoL) and effective treatments with less toxicity. Prof Mathias Rummel discussed modern methods of assessing FL risk and predicting treatment outcomes, particularly regarding endpoint selection for clinical trials. Dr Andrew Davies presented data from the GALLIUM study, showing that obinutuzumab-chemotherapy and maintenance is superior to rituximabchemotherapy and maintenance in untreated advanced FL patients, while Prof Gilles Salles provided insight into future options being developed for patients with FL.
Management Issues in Follicular Lymphoma
Professor Bruce Cheson
Traditionally, FL was thought an indolent, slow-growing disease that is likely to respond to treatment, when necessary. However, in 2015, Casulo et al.1 identified two groups of patients with FL: those who do not relapse within 2 years and have survival outcomes equivalent to an age-matched population, and those who relapse within 2 years and have a long-term disease-free survival rate of only 20–30%.
While a number of treatment options are available for patients who relapse, each has major limitations, particularly surrounding safety. Therefore, a number of new targeted therapies have been developed including molecules that interact with the cell surface, such as monoclonal antibodies (mAb) and antibody drug conjugates, those that inhibit intracellular pathways, and those which affect the microenvironment. However, it is still difficult to identify patients who are likely to relapse and manage them accordingly. So, novel methods of risk-stratifying patients with FL may improve the accuracy of a patient’s prognosis prior to treatment and allow risk-adaptive clinical trials to be conducted, leading to proactive treatment rather than reactive management of patients who relapse.
The Patients’ Perspective and Quality of Life in Follicular Lymphoma
Ms Rosmarie Pfau
The Lymphoma Coalition (LC) is an international network of patient groups that has created a global collection of useful resources, including a database of therapies and clinical trials by subtype and country and information from patient surveys. The database now contains a resource library of 2,500 pieces of information, such as lymphoma abstracts, diagnostic guidelines, best practice approaches, and data from large patient surveys.
The 2016 LC Global Patient Survey had 4,154 respondents, including 778 with FL.2 The aim of the survey was to better understand the patient experience and to assess how treatment-related issues affect QoL. Notably, patients indicated that treatment for FL is associated with various toxicities, for example:2
- 13% of survey respondents reported gastrointestinal issues
- 16% of survey respondents suffered tingling in the extremities
- >20% of survey respondents experienced numbness
These issues strongly influence a patients’ QoL, especially as their impact lingers, even after discontinuing treatment (Figure 1). For example, >25% of survey respondents experienced treatment-related issues for 3–5 years, and almost 15% of respondents still experienced treatment-related toxicity 8 years later.2
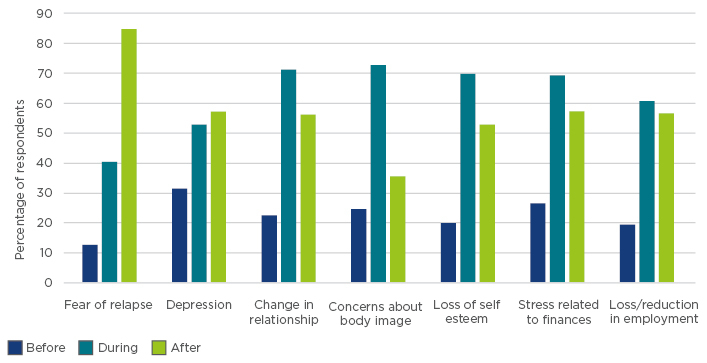
Figure 1: Effect of treatment on quality of life of survey respondents with follicular lymphoma.2
Fatigue is the most commonly reported physical condition, affecting >70% of respondents, and can have a profound influence on functional performance, mood, and overall QoL.2 Importantly, >80% of patients replying to the survey experienced a fear of relapse during or after treatment. Depression is also common, which can impact the course of the disease and treatment compliance.2 In particular, patients in the ‘watch and wait’ situation live under a near-constant cloud of fear.2 Healthcare professionals should refer patients with FL to patient organisations to ensure patient access to accurate information and an experienced support network.
Patients with FL are generally receptive to treatment and tend to prioritise therapeutic efficacy over QoL initially, because they are intent on living as long as possible.2 Therefore, it can sometimes be difficult for patients to understand the medical reasoning behind a watch and wait strategy. However, after experiencing adverse events (AE) associated with treatment, many patients will prioritise QoL over efficacy and see value in treatment-free time, because subsequent rounds of chemotherapy make patients feel as though they are being ‘kicked while they are down’. The main wish for patients was for effective treatments with less toxicity.2 Patients with FL often depend on information provided by healthcare professionals, but may have difficulty understanding medical terminology; this information is critical, as it may affect how they choose to manage their disease. However, patient organisations can help provide additional information, education, and support to healthcare professionals and answer questions from patients with FL.
In summary, the LC Global Patient Survey demonstrated that patients require additional support beyond what they are currently receiving.2 Patient organisations are available to support patients in addition to healthcare professionals, but, in most countries, patients are not actively directed to these organisations. As such, patient organisations must aim to build stronger relationships with healthcare professionals, while continuing to connect with and support patients.
For the full talk by Ms Rosmarie Pfau click here.
How can we Improve First-line Treatment for Follicular Lymphoma and Measure This Improvement?
Professor Mathias Rummel
FL represents the largest subtype of indolent non-Hodgkin’s lymphoma (NHL)3 and its optimal management is widely debated and variable across regions. Nevertheless, the fact remains that >85% of patients eventually require systemic treatment.3-6 A wide variety of therapies are available for FL, including radiotherapy and single-agent or combination chemotherapy. The addition of anti-CD20 mAb rituximab to induction chemotherapy has significantly improved progression-free survival (PFS), overall survival (OS), and time to treatment failure in patients with untreated NHL.7-11 Maintenance therapy with 2 years of rituximab monotherapy can also be used following a response to induction therapy, which significantly improves PFS, although with no impact on OS.12
In summary, out of 100 patients requiring first-line treatment, approximately 10% of patients will not respond. Overall, 50% of patients will stay in remission for more than 10 years, and nearly 50% of patients will progress or die within 6 years.13,14 Ideally, risk-stratification criteria or biomarkers could be used to identify those patients with FL who are at risk of poor outcomes.
Assessment of Risk in Follicular Lymphoma
The Follicular Lymphoma International Prognostic Index (FLIPI), and its successors FLIPI-2 and m7-FLIPI, were developed as risk assessment tools for patients with FL.15-18 Studies have demonstrated a significant association between disease progression within 24 months (POD24) and inferior OS, even after adjustment for FLIPI risk score.1,18,19 The m7-FLIPI, which incorporates the mutational status of seven genes, outperforms both FLIPI and FLIPI-2 for risk prognostication (Figure 2).17,18 The m7-FLIPI also predicts POD24 more accurately than FLIPI and is prognostic for failure-free survival/OS in patients without POD24.18
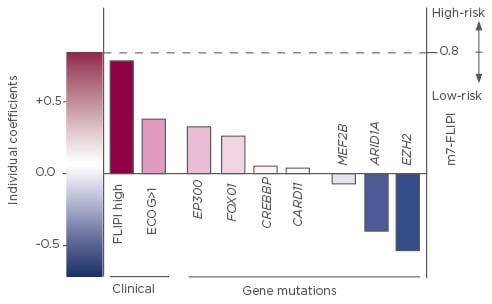
Figure 2: Components and relative coefficients used in the calculation of the m7-FLIPI.17
FLIPI: Follicular Lymphoma International Prognostic Index; ECOG: Eastern Cooperative Oncology Group performance status.
Potential Surrogate Endpoints for Overall Survival and Progression-Free Survival
While OS provides an objective measure of treatment efficacy, its use as a primary endpoint requires a long duration of follow-up, which would prolong the process of identifying novel and potentially beneficial therapies. Therefore, time-to-event endpoints, such as event-free survival and PFS, are increasingly being used as primary endpoints in NHL studies. For example, increased PFS translated into a significant improvement in the time to next treatment, even after 10 years follow-up, representing a considerable benefit for patients.10 However, there was no significant difference in OS, which is likely due to improvements in second and third-line treatment options.10
It should be noted that PFS also requires extended follow-up for patients with FL, because the median PFS for FL can extend beyond 7 years. Therefore, several surrogate endpoints have been suggested, including:
- Complete response rate at 30 months (CR30): An individual patient-level analysis of multiple randomised trials, conducted using patient data for >3,800 patients, found a minimum 11% absolute improvement in CR30 from a 50% control rate and predicted a significant treatment effect on PFS (hazard ratio [HR]: 0.69).20
- Positron emission tomography-computed tomography (PET-CT): The predictive power of PET-CT is stronger than FLIPI, FLIPI-2, and CT-based response assessments and minimal residual disease (MRD) at the end of induction.21
- MRD negativity: MRD negativity has been associated with prolonged PFS, event-free survival, and freedom from recurrence, but not OS.22-26 MRD status at diagnosis/screening may also predict response and/or long-term outcomes.27-29 Combining MRD with other prognostic markers, such as PET-CT, may therefore improve the ability to predict disease progression.30
- Baseline total metabolic tumour volume: This measure can be used to identify patients with FL at high risk of progression. Patients with high total metabolic tumour volume (>510 cm3) had significantly shorter PFS and OS, even after adjusting for induction treatment.31
Summary
While a ‘watch and wait’ approach can be used for some patients with FL, most patients eventually require treatment with an anti-CD20 mAb in combination with chemotherapy once diagnosed. Any response achieved may also be followed using post-induction maintenance to prolong PFS.
However, given the significant time lag between diagnosis and progression or survival events in patients with FL, surrogate endpoints may expedite the development of new therapies. Likewise, new risk stratification tools are being developed and will be investigated in clinical trials in an attempt to more effectively target therapies to those patients who are most likely to benefit.
For the full talk by Professor Mathias Rummel click here.
Recent Advances in Treatment for Patients with Follicular Lymphoma
Doctor Andrew Davies
In the last few years, greater insight has been gained into the biology of FL, generating a range of new prognostic and predictive tools, as well as new therapies. However, integrating the whole range of novel agents that affect the tumour microenvironment, dysregulated pathways, and cell surface antigens into treatment pathways for FL is an ongoing challenge.
The current therapeutic algorithm for FL recommended in the European Society for Medical Oncology (ESMO) guidelines suggests watching and waiting for asymptomatic patients who have minimal symptoms or using a less intensive treatment approach, such as single agents or rituximab.32 For symptomatic patients with a high tumour burden, immunochemotherapy may be offered.32
Obinutuzumab: A New Anti-CD20 Treatment in Follicular Lymphoma
Obinutuzumab is a Type II, third-generation anti-CD20 mAb with enhanced antibody-dependent cellular cytotoxicity over rituximab and increased direct cell death activity that has now been included in the National Comprehensive Cancer Network (NCCN) guidelines.33 In the largest Phase III study ever conducted in FL (GALLIUM),34 1,202 patients with Stage III/IV, or bulky Stage II, Grade 1–3a FL indicated for first-line treatment were randomised to treatment with rituximab or obinutuzumab in combination with cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP), cyclophosphamide, vincristine, and prednisone (CVP), or bendamustine chemotherapy.34 Patients who responded to treatment continued maintenance antibody monotherapy according to the arm that they had been randomised.34
The primary endpoint of investigator-assessed PFS was reported after the Data Monitoring Committee recommended release of data after the planned interim analysis. A 34% reduction in the risk of death or progression after 34 months of follow-up was reported for patients treated with obinutuzumab and chemotherapy versus rituximab and chemotherapy (3-year PFS: 80.0% versus 73.3%, respectively; HR 0.66; 95% confidence interval [CI]: 0.51–0.85; p=0.012; Figure 3).34 A supporting outcome of a 29% reduction in events was found following the Independent Review Committee’s assessment of PFS (3-year PFS: 81.9% versus 77.9%; HR 0.71; 95% CI: 0.54–0.93]; p=0.0138). No difference in OS or objective response rate (ORR), as defined by CT scan, was observed in the interim analysis.34
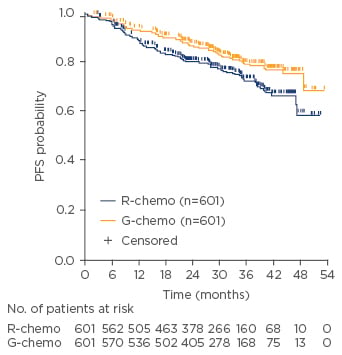
Figure 3: Investigator-assessed progression-free survival for patients treated with obinutuzumab plus chemotherapy versus rituximab plus chemotherapy.34
G-chemo: obinutuzumab plus chemotherapy; PFS: progression-free survival; R-chemo: rituximab plus chemotherapy.
The overall proportion of patients experiencing AE was comparable between study arms, but a higher number of Grade ≥3 events were reported for patients treated with obinutuzumab.34 In particular, the prevalence of neutropenia, infusion-related reactions, and infections was higher in the obinutuzumab arm, but the difference in the number of fatal AE between arms was not statistically significant.34 Fatal AE were more common in patients treated with bendamustine combination treatment, most notably as a result of infection.34 Obinutuzumab was also associated with a slightly higher prevalence of secondary malignancies.34 A greater proportion of patients treated with obinutuzumab achieved MRD negativity at mid-induction compared with rituximab, which was maintained through to the end of induction, independently of the backbone chemotherapy regimen.35 Importantly, PFS was higher for patients who achieved MRD negativity versus those who did not, particularly for patients treated with obinutuzumab.35,36
Patients with FL that relapse early after rituximab and chemotherapy or rituximab monotherapy have a poor outcome and a high unmet medical need. In order to improve treatment outcome for these patients, the efficacy and safety of obinutuzumab in combination with bendamustine (versus bendamustine alone) were also investigated in 413 patients with indolent NHL, 335 with the diagnosis of FL, who were refractory to rituximab therapy (GADOLIN).37 As observed earlier, there was no difference in the end of induction response rates between the two arms of the studies. The addition of obinutuzumab to bendamustine was found to increase PFS, the primary endpoint of this study, for patients with FL by 11.3 months after 31.2 months of follow-up (median PFS: 25.3 versus 14.0 months; HR: 0.52; 95% CI: 0.39–0.69; p<0.0001).37,38 The prolonged PFS translated into a meaningful difference in OS in the combination arm (median OS not reached versus 53.9 months; HR: 0.58; 95% CI: 0.39–0.86; p=0.0061).38 The AE profile showed no new safety signals.38 MRD negativity could be achieved at a greater rate in those patients treated with the obinutuzumab and bendamustine combination compared with those receiving bendamustine monotherapy (82% versus 43%).36 As in GALLIUM, reaching MRD negativity was associated with prolonged PFS.36
Summary
Obinutuzumab is more efficacious than rituximab when combined with chemotherapy in the initial treatment of FL. Discussions around toxicity, particularly surrounding treatment with anti-CD20 therapy and bendamustine, should be held with individual patients. Further advances in treatment are likely to result from a better understanding of the underlying disease biology for patients with FL.
For the full talk by Doctor Andrew Davies click here.
Future Treatments for Patients with Follicular Lymphoma
Professor Gilles Salles
Despite the efficacy of treatments for FL in achieving early remission, a number of patients only experience a short-term solution. Relapse or ongoing toxicities can have an ongoing adverse effect on patient QoL, so it is imperative that new treatments are developed that offer patients greater choice and improved clinical outcomes.
Naked Antibodies (Anti-CD20 and Others)
mAb offer antigen-specific targeting of lymphocyte markers, such as CD20, and promote tumour destruction through immune-cell recruitment. Rituximab was the first anti-CD20 mAb approved for use in patients with FL, and has subsequently become a standard treatment option, either as monotherapy, or in combination with chemotherapy or immunomodulatory agents. More recently, ‘new-generation’ mAb, such as obinutuzumab, have been developed that offer prolonged PFS compared with rituximab.34,38
Bi-specific Antibodies and Antibody Drug Conjugates
Advances in manufacturing techniques have also encouraged the development of antibody derivatives that may offer advantages over their ‘naked’ mAb counterparts. For example, bi-specific antibodies (e.g. blinatumomab) are capable of targeting two separate antigens, localising and activating immune cells to their tumour targets. In addition, mAb can be used to directly deliver cytotoxic agents to tumour cells as antibody-drug conjugates (ADC). Polatuzumab vedotin is an ADC that delivers monomethyl auristatin E to CD79b+ B cells, mediating cell death via microtubule disruption.39 While the clinical efficacy of polatuzumab vedotin is yet to be established, initial Phase I/II trials suggest positive outcomes for patients treated with polatuzumab vedotin in combination with rituximab.40
Immune Checkpoint Inhibitors
Some tumours produce ligands capable of dampening the patient’s immune response.41 For example, tumour cells expressing programmed death-ligand 1 (PDL-1) engage programmed death-1 (PD-1) receptors on activated T cells to avoid immune-mediated cell death.42 Accordingly, immunotherapy can be used to disrupt this tumour cell survival mechanism, and a 40% ORR (but with a short response duration) has been observed in a Phase I study of the anti-PD-1 mAb nivolumab in patients with extensively pretreated FL.43
Immunomodulatory Imide Drugs
Immunomodulatory imide drugs are another class of promising agents that exhibit direct antineoplastic activity, in addition to modulating immune cells in the tumour microenvironment.42 Lenalidomide was well tolerated in an initial randomised controlled trial in combination with rituximab (the ‘R2’ regimen), conferring a 3-year PFS of 78.5% (95% CI: 66.8–92.2%) in previously untreated patients.44 The Phase III RELEVANCE trial is assessing the efficacy of R2 compared with standard chemotherapy, while the AUGMENT study is comparing R2 with rituximab monotherapy.45,46
Targeted Therapies
Targeted therapies offer a more direct treatment option, using small molecules capable of directly interfering with pathways essential to tumour growth and progression. For instance, phosphatidylinositol-4, 5-bisphosphate 3-kinase, and Bruton’s tyrosine kinase inhibitors, such as idelalisib and ibrutinib, can reduce tumour size and might improve OS in patients with relapsed or refractory FL.47,48 However, certain subsets of patients display resistance to these targeted therapies, and each treatment presents a different safety profile. Apart from kinase inhibitors, the apoptosis-inducing B cell lymphoma 2 (BCL-2) inhibitor venetoclax, has shown activity in a Phase I study. An ORR of 34% was achieved in patients with FL, although the complete response rate was only 14%.49 In addition, molecules are currently in development that target EZH2, a histone methyl transferase associated with the maturation of B cells; activating mutations in EZH2 are present in 20–25% of follicular and diffuse large B cell (germinal center B cell subtype) lymphomas.50 Early Phase I results with tazemetostat demonstrate this may be a promising agent for treatment of FL.51
Chimeric Antigen Receptor T cell
Finally, preliminary results from studies with chimeric antigen receptor T cell therapy suggest that, despite the potential toxicities associated with its use, it may be a very active treatment with long-standing remission for patients with FL.52
Future Therapeutic Options
Currently, effective treatment of FL is hindered by the high degree of heterogeneity between tumour cells, in combination with underlying differences in the tumour microenvironment between individual patients. Therefore, future investigations should focus on developing therapeutic combinations that are potentially active in ‘all’ B cell lymphomas (e.g. compounds active on ‘the immune response’, ADCs, or chimeric antigen receptor T cell therapy) or which focus specifically on selected subsets of FL.
For the full talk by Professor Gilles Salles click here.
Conclusions
While patients with FL respond to treatment and have relatively long survival compared with other cancers, treatment can have a lasting impact on QoL. Early relapse is associated with a poor outcome, and the fear of relapse is an ongoing psychological burden for many patients with FL. Of note is the difficulty in investigating novel treatment strategies for FL, due to the long-dated endpoints and heterogeneous nature of the disease. The development of new risk stratification tools and increasingly targeted therapies will not only reduce toxicities, but may also improve survival outcomes for patients with FL.
Click here to view the full symposium.







