Meeting Summary
This continuing medical education-accredited symposium, held at the 2023 International Society for Thrombosis and Haemostasis (ISTH) congress in Montréal, Canada, focused on current unmet needs in anticoagulation, especially in the atrial fibrillation (AF) population, and reflected on the promise of the emerging class of Factor XI inhibitors for stroke prevention (SPAF) in susceptible patients. The faculty agreed that, although direct oral anticoagulants (DOAC) have represented a major advance compared with vitamin K antagonists, their utilisation remains suboptimal, often due to the prevailing fear of bleeding in many types of patients. Older age alone can be a reason for withholding anticoagulation, due to the risk and implications of bleeding. Frailty and comorbidities, such as chronic kidney disease (CKD), which can adversely affect the bioavailability of DOACs, are also deterrents to optimal anticoagulant use. Clinicians may try to avoid or mitigate bleeding by inappropriately prescribing low doses of DOACs, an off-label practice that has been found to fail to protect patients from thrombotic risk, without attenuating the risk of bleeding. In addition, the potential for drug-drug interactions and poor adherence also limit the optimal use of DOACs in real-world clinical practice. A recent patient survey focusing on the topic of ‘minor bleeding’, often referred to by clinicians as ‘nuisance bleeding’, and typically not well captured in clinical trials, revealed the far-reaching impact of ongoing problems with bleeding on quality of life, and the possibility that these experiences may deter patients from adherence to their prescribed anticoagulant regimen. Factor XI represents a promising new target for anticoagulation, which may minimise the risk of bleeding by pharmacologically ‘uncoupling’ the clotting pathway, leading to pathological thrombosis from the cascade largely responsible for physiological haemostasis. Phase II research with investigational Factor XI inhibitors has established their antithrombotic and safety potential, and some of these agents may also avoid other practical drawbacks of DOACs. Phase III evaluation of Factor XI inhibition is ongoing in a number of clinical settings.
Introduction
Shaun G. Goodman
Shaun G. Goodman, Canadian VIGOUR Centre, University of Alberta, Edmonton, Canada, and St Michael’s Hospital, University of Toronto, Canada, set the scene by reminding the audience that thromboembolism is responsible for approximately one in four deaths globally, and remains a leading cause of morbidity.1 A key contributor to this immense burden of disease is the widespread underutilisation of evidence-supported anticoagulation regimens, a situation largely driven by fear of bleeding.1 Although the DOACs (apixaban, dabigatran, edoxaban, and rivaroxaban) have proved more convenient to administer than vitamin K antagonists (VKA), such as warfarin, and have a better safety record, especially in regard to intracranial haemorrhage, the risk of bleeding remains a concern.2 A 2014 meta-analysis showed that, despite a marked reduction in all-cause mortality with DOACs versus VKAs, annual rates of major bleeding, and the composite of major or clinically relevant non-major (CRNM) bleeding in elderly patients with AF were notable, at 5% and 12%, respectively.2 Moreover, randomised clinical trials comparing individual DOACs with VKAs reported that, in patients on DOACs experiencing major bleeds, the fatality rate remained 7–10%.3-6 A similar rate of fatal major bleeds (5.1%) was observed in a real-world DOAC registry.7
Goodman went on to observe that clinical trials have generally captured only major bleeding and CRNM bleeding, so the impact on patients of the totality of bleeding from anticoagulation remains to be fully understood. For example, if frequent nosebleeds, often referred to by clinicians as ‘nuisance bleeding’, cause a patient to pause or discontinue their anticoagulant, it should be questioned whether such events can really be considered ‘minor’, or merely ‘a nuisance’ to them. Beyond the formidable challenge of bleeding, currently available anticoagulants have various incompatibilities with patients’ typically complex comorbidities and concomitant medications, as well as well-demonstrated issues with adherence and persistence. Thus, although the DOACs represent a major advance in anticoagulation strategy over VKAs, substantial unmet needs remain.8
Current Challenges in Anticoagulation in Higher Risk Patients
Jean Connors
Challenges of Prescribing Anticoagulants in the Atrial Fibrillation Population
Jean Connors, Brigham and Women’s Hospital, Dana-Farber Cancer Institute, and Harvard Medical School, all based in Boston, Massachusetts, USA, began by establishing that a key patient population in particular need of safe and effective anticoagulation is the AF population, which represents 65–70% of the 3,600 patients cared for by the Brigham and Women’s Hospital anticoagulation management service in Boston. Connors highlighted the high and growing global prevalence of AF, which amounts to over 37 million cases, and is predicted to increase by as much as 60% by 2050.9,10 The size of this population means that the burden of complications exerts a significant impact on healthcare provision, and society in general. Since AF is well known to greatly increase the risk of stroke, anticoagulant protection is especially important in this population. Stroke risk prediction tools, such as the CHA2DS2-Vasc score can help identify those individuals at low and high risk of stroke to help guide decision-making around anticoagulant prescribing; however, scores used to predict bleeding risk once therapy is initiated are less robust. The most validated of these is the HAS-BLED score, but this was developed in patients on warfarin; efforts to develop bleeding risk scores in the DOAC era have achieved only modest predictive value for major bleeding.11 Thus, anticoagulant prescribing in AF currently remains a precarious balancing act, with the aim of minimising the risk of stroke and other thromboembolic events without unduly raising the risk of bleeding.
Evolution of Anticoagulant Options and Prevailing Underuse of Direct Oral Anticoagulants
Reviewing a timeline of developments in anticoagulant therapy over the years, Connors commented that progress was initially very slow, with unfractionated heparin and warfarin being the only options for decades. The arrival of low molecular weight heparins in the 1980s was an important milestone, but it was not until the approval of the DOACs in the 2000s that the pace of progress really began to accelerate. In many ways, DOACs have transformed the practice of anticoagulation, avoiding the need for international normalised ratio monitoring and consequent dose calibration that makes warfarin prescribing burdensome for patients and healthcare teams, and expensive for the care system.
Nonetheless, DOACs are clearly not without shortcomings, a reality that is reflected by their frequent underuse.12-14 A recent USA database analysis of Medicare beneficiaries aged ≥65 years showed that, within 12 months of a new AF diagnosis, 67% of patients were not given any anticoagulation, despite their evident risk of stroke, and the widespread availability of DOACs.12 The withholding of anticoagulation in such a high proportion of people is a serious concern, which highlights the clinician anxiety that still prevails around safe anticoagulant prescribing. The reasons clinicians gave for their decision not to prescribe anticoagulation in the Medicare database study12 included older age of the patient, which, Connors observed, is going to become increasingly difficult to justify as the general population ages (Figure 1). Another reason given was pre-treatment anaemia that, Connors reflected, is perhaps associated in the minds of prescribers with a likelihood of poorer outcomes if patients bleed, or by the potential to exacerbate unrecognised ongoing indolent bleeding, such as with a gastrointestinal malignancy. A third reason given was patient frailty, which is generally recognised as an indicator for adverse events in any medical discipline, though it is not usually identified by standard metrics. A fourth reason, dementia, needs little explanation, but the final reason, CKD, is a major and increasing problem when prescribing anticoagulants, and an area where DOACs, which require daily or twice-daily dosing, may be considered potentially unsafe.
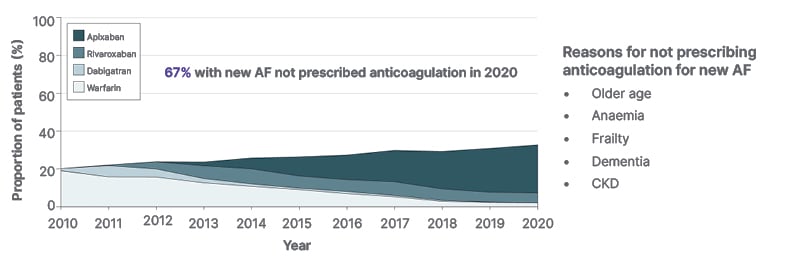
Figure 1: Oral anticoagulant initiation and direct oral anticoagulant uptake in 2010–2020.12
12-month OAC initiation and direct OAC uptake in the total OAC-eligible incident atrial fibrillation cohort.
OAC initiation increased from 20.2% to 32.9% (odds ratio for OAC use per year: 1.06; 95% CI: 1.06–1.07; p<0.001).
AF: atrial fibrillation; CI: confidence interval; CKD: chronic kidney disease; OAC: oral anticoagulants.
The Challenge of Chronic Kidney Disease in Patients Eligible for Anticoagulation
Over one-third of patients with AF also have some degree of CKD, with both conditions related to ageing.15 The presence of CKD in the AF population is strongly associated with poorer clinical outcomes (both stroke/systemic thromboembolism and bleeding).16 For approximately 40% of patients with AF, impaired renal function is sufficiently severe as to pose concerns for the use and dosing of anticoagulants.14 In regard to the renal clearance of different DOACs, dabigatran (a Factor II inhibitor) is 80% renally cleared which, Connors recalled, led to a surge in emergency department visits for bleeding when it was first introduced.17 This was due to unexpected declines in patients’ creatinine clearance, which precipitated a marked increase in plasma dabigatran levels due to bioaccumulation. With the other DOACs (Factor Xa inhibitors), renal clearance ranges from approximately 50% with edoxaban to approximately 27% with apixaban.17 But even patients on apixaban may have unforeseen decline in renal function that affects their plasma drug concentration, resulting in elevated anticoagulation intensity, and a heightened risk of bleeding. Thus, the co-existence of CKD, an escalating global health issue, significantly complicates the use of DOACs in people who need anticoagulation.
Potential Drug-Drug Interactions with Direct Oral Anticoagulants
A further concern when prescribing DOACs is the possibility of major drug–drug interactions. Some patients eligible for anticoagulation are at risk of such interactions with concomitant medications that affect cytochrome P450 3A4 or P-glycoprotein metabolism, which include antibiotics such as rifampicin, antifungal agents, antiseizure medications, and, importantly, many modern targeted anticancer drugs. Connors observed that patients with cancer in particular are frequently concerned about taking medications that might interfere with the efficacy or safety of their cancer treatment. A population-based Canadian study, looking at over 642,000 DOAC prescriptions in over 36,500 patients with AF, found concomitant prescribing of a P-glycoprotein- or cytochrome P450 3A4-metabolised drug in a relatively small proportion of patients (11.2%), but when an adverse drug–drug interaction was reported, inappropriate DOAC dosing was noted in 63% of cases, with a 1.6-fold higher risk of death at 1 year.18 Thus, there are specific cohorts of patients for whom DOACs may not be appropriate for this reason. It is also worth noting that understanding of potential drug-drug interactions with DOACs is incomplete, and some individuals take numerous drugs for multiple conditions, where potential interactions could multiply with unpredictable results.
Inappropriate Dose Reduction of Direct Oral Anticoagulants
In an attempt to minimise the risk of bleeding, clinicians often prescribe inappropriately low doses of DOACs.19-23 A study conducted among inpatients at the Brigham and Women’s hospital, where Connors practices, found a reduced dose regimen in 13% of 224 patients receiving DOACs.19 Pharmacist-led assessment of the renal function of these patients, alongside the package label of the DOAC prescribed, found that the reduced dose was justified in fewer than 50% of these individuals. Importantly, those on an off-label dose reduction still experienced a roughly equal number of thrombotic events (20%) and bleeds (25%), meaning that they experienced loss of anticoagulant efficacy without greater safety. This finding was borne out by a much larger analysis of 7,577 patients in the Outcomes Registry for Better Informed Treatment (ORBIT), whose bleeding risk scores were known. Of those receiving a standard dose of DOAC, only 4% were found to be inappropriate, but of those receiving a reduced dose, 57% were deemed inappropriate.20 Finally, in an analysis of almost 15,000 patients with AF, 4% had an inappropriate standard-dose DOAC prescription (i.e., their dose had not been reduced despite having a renal indication for dose reduction) but three times as many (12%) were found to have an inappropriately reduced dose, i.e., they did not meet package label criteria for dose reduction.21 Moreover, dose reduction in apixaban-treated patients in this study was found to be associated with an increased risk of stroke without a reduced risk of bleeding.21
Implications of Poor Adherence with Daily Oral Drugs
In addition to undertreatment, the effectiveness of DOACs, which patients need to take orally on a daily basis due to the pharmacokinetic properties of these drugs, can be jeopardised by poor adherence.24-26 In a review of 48 real-world studies, patients with AF missed a DOAC dose once every 4 days.26 Poor adherence was associated with a 39% increased risk of thromboembolic events.26 Because of the short half-life of DOACs (5–14 hours), even one missed dose can place a patient in a ‘low protection’ zone (Figure 2).27-29
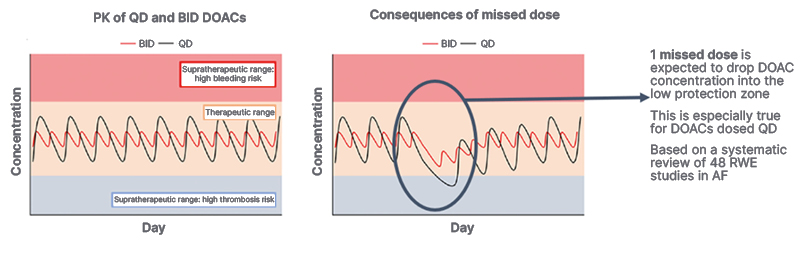
Figure 2: Consequences of missed doses of direct oral anticoagulants.27,28
AF: atrial fibrillation; BID: twice per day; DOAC: direct oral anticoagulants; PK: pharmacokinetics; QD: once per day; RWE: real-world evidence.
Summarising, Connors acknowledged the advantages of DOACs compared with previous anticoagulant options, but also noted that all of the prevailing concerns from the warfarin era have not been eliminated with DOACs. Many patients at risk of thromboembolism are still untreated or undertreated, and clinicians remain worried about how to manage DOAC prescribing in the elderly and those with reduced kidney function. As for adherence, this remains a problem for all daily oral drugs in preventative settings, and the DOACs for SPAF, which afford no symptom relief but may be associated with bruising and other bothersome bleeding events, are no exception.
The Promise of Factor XI Inhibition
Jeffrey Weitz
Jeffrey Weitz, Thrombosis & Atherosclerosis Research Institute (TAARI), McMaster University, Hamilton, Canada, began by reiterating current unmet needs in anticoagulation, particularly for SPAF, emphasising that the ultimate goal when prescribing an anticoagulant is to attenuate thrombosis risk without meaningfully increasing the risk of bleeding. Although the DOACs come closer to this goal than VKAs, the risk of bleeding with DOACs remains concerning, contributing to their systemic underuse. Ultimately, many patients who need anticoagulant protection are not receiving it, highlighting the need to explore new approaches to SPAF.
Rationale for Factor XI as a New Target for Anticoagulation
Weitz went on to explain the rationale for focusing on Factor XI as a promising new target for anticoagulation, stating that the evidence to support this comes from several sources. Firstly, it has been observed that people with severe congenital Factor XI deficiency appear to be protected from thrombosis, but very rarely have serious or spontaneous bleeding.30 Secondly, large genetic epidemiology studies have shown that, compared with individuals with normal Factor XI levels, people with low Factor XI levels have a reduced risk of thrombosis, while people with high Factor XI levels are at increased risk of thrombosis.31,32 Finally, numerous animal models in rodents and non-human primates indicate that inhibition of Factor XI attenuates both venous and arterial thrombosis with no increase in bleeding.33 This is a very different outcome from that seen in animal models with DOACs where thrombosis was also attenuated, but the bleeding rate increased in line with increasing dosage.
The Prospect of ‘Uncoupling’ the Pathways of Thrombosis and Haemostasis
Weitz described our understanding that Factor XI inhibition appears able to prevent thrombosis without simultaneously impacting haemostasis, a finding inconsistent with traditional teaching about how the coagulation cascade works. The conventional depiction of the coagulation cascade suggests that the pathways leading to pathological thrombosis and physiological haemostasis are inextricably linked. Essentially, the thinking was that, by blocking fibrin, we inevitably intervene not only in harmful clotting, but also in helpful clotting, leading to the long-held belief that it is impossible to achieve effective anticoagulation without an appreciably increased bleeding risk.
However, a newer model of the coagulation cascade, informed by insights from genetic, epidemiological, and animal studies, has now emerged.34 It reveals two distinct pathways, with only one section in common: the downstream ‘common’ pathway. The pathway of physiological haemostasis, also known as the extrinsic or tissue factor pathway and measured readily by the prothrombin time, leads to the formation of extravascular haemostatic ‘plugs’ that seal leaks and injuries in vessel walls to prevent bleeding. In contrast, pathological thrombosis results from the generation of an intravascular clot that ultimately occludes the flow of blood within arteries, leading to heart attacks and strokes; or within veins, leading to deep vein thrombosis and pulmonary embolism. This process might also initiate with tissue factor, but the growth of pathologic thrombi, to an extent sufficient to occlude blood vessels (which occurs via the intrinsic pathway, and is measured by the partial thromboplastin time), depends on an amplification loop driven by Factor XI.
Weitz further explained that, if we consider the targets of currently available anticoagulants, it becomes clear that the vitamin K-dependent factors targeted by warfarin are located in both of these pathways, while Factor Xa and thrombin, targeted by DOACs, reside in the shared ‘common pathway’ (Figure 3A). This explains why these approaches to anticoagulation, while protecting against thrombosis, also undermine haemostasis, which can lead to bleeding. In contrast, Factor XI is situated only in the amplification loop of the intrinsic, pathological thrombosis pathway. It is not involved in haemostasis, but is essential for the promulgation of harmful clotting that leads to thrombus growth and vessel occlusion (Figure 3A). Thus, by targeting Factor XI, we can conceptually ‘uncouple’ the haemostatic pathway from the pathologic thrombosis pathway (Figure 3B).34 Leaving the haemostasis pathway intact enables us, in theory, to inhibit thrombosis with a potentially minimal risk of bleeding, a vision that has long been seen as the ‘holy grail’ of anticoagulation therapy.
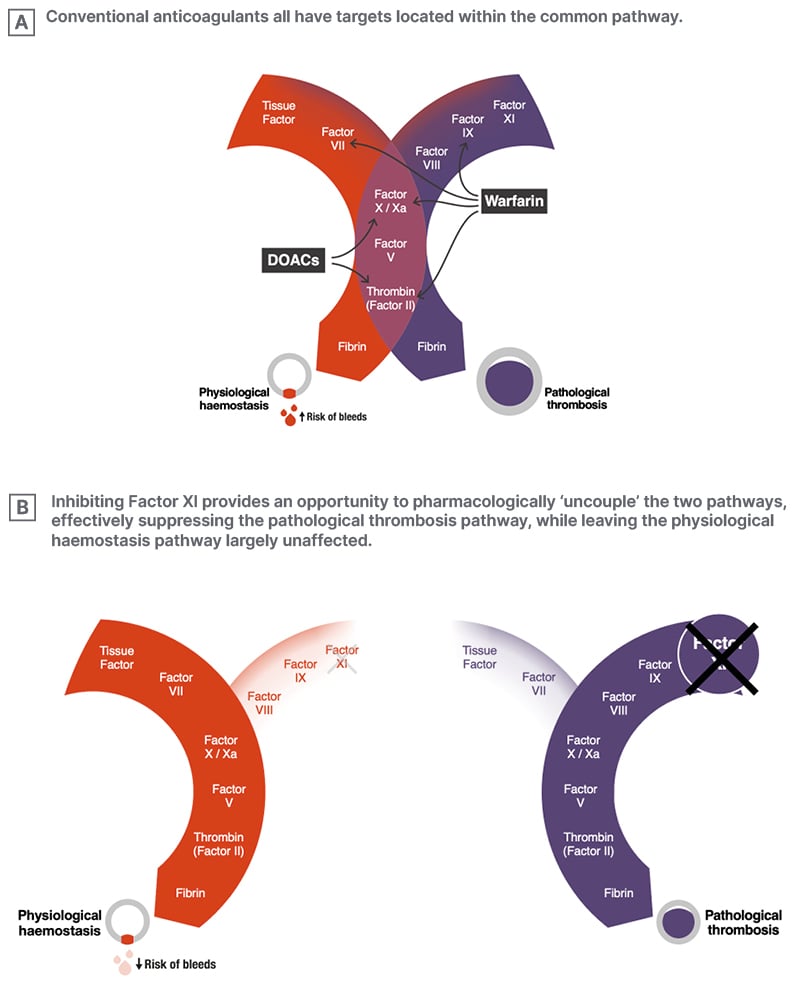
Figure 3: A new model of the coagulation cascade reveals the promise of Factor XI inhibition.34
DOAC: direct oral anticoagulant.
Current Approaches to Factor XI Inhibition
Weitz went on to review the various diverse modalities that have emerged to target Factor XI. The monoclonal antibodies abelacimab and osocimab both inhibit Factor XI but in different ways: abelacimab binds to zymogen Factor XI (the inactive precursor), blocking its activation to Factor XIa. In contrast, osocimab binds directly to the activated form, Factor XIa. Fesomersen, a second-generation antisense oligonucleotide, reduces the synthesis of Factor XI by targeting its messenger RNA in the liver. Finally, the small molecules asundexian and milvexian bind to the active site of Factor XIa to block its activity, much as the DOACs do with Factor Xa. All of these strategies have the potential to achieve anticoagulation with a lower risk of bleeding compared with current options. However, the monoclonal antibody Factor XI inhibitors may have additional benefits. These can be administered intravenously in acute care settings, to achieve a rapid onset of action, or as a once-monthly subcutaneous regimen for long-term community use, which is likely to be supportive of improved adherence. In addition, there is no dependence on renal clearance, and minimal risk of drug–drug interactions with these agents (Table 1).1,35
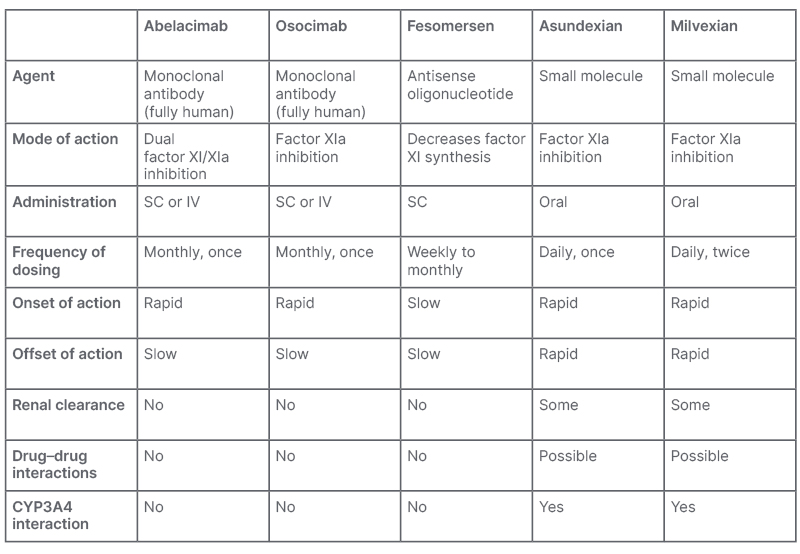
Table 1: Mechanistic differences between investigational Factor XI inhibitors.1,35
CYP3A4: cytochrome P450 3A4; IV: intravenous; SC: subcutaneous.
Phase II Data with Investigational Factor XI Inhibitors
Weitz then presented the Phase II data published to date with several of these investigational agents. With the exception of asundexian, all Factor XI inhibitors have been compared with standard of care enoxaparin for prevention of venous thromboembolism after knee replacement surgery. This is the gold standard model for evaluating the efficacy of potential new anticoagulants because patients undergoing knee replacement surgery are at high risk for venous thromboembolism, which can easily be measured, even when asymptomatic, through venography (X-ray of the veins of the operated leg). A meta-analysis of results of these Phase II studies showed an average 40% reduction in the rate of venous thromboembolism with the Factor XI inhibitors compared with enoxaparin, establishing the antithrombotic efficacy of the class.36 Although these studies were not designed to assess safety, since the absolute risk of bleeding is low with this type of surgical procedure, promising reductions in major and CRNM bleeding compared with enoxaparin were observed, and additional Phase II research is underway to investigate this further.
Phase III Evaluation of Investigational Factor XI Inhibitors
The promise of Factor XI inhibitor therapy has prompted the initiation of a range of Phase III trials, which in aggregate are planned to enrol 78,000 patients. The small molecules asundexian and milvexian are being compared with apixaban in patients with AF, and are also being studied in secondary stroke prevention (and, in the case of milvexian, in acute coronary syndrome) in combination with antiplatelet therapy. In contrast, the LILAC trial is studying abelacimab in a special subgroup of patients with AF: those deemed clinically unsuitable for any current anticoagulant, who have the most to gain from a potentially safer option. Abelacimab is also being studied in cancer-associated thrombosis, an area of growing importance and recognition.
In conclusion, Weitz reiterated that Factor XI is a promising new target that could revolutionise the practice of anticoagulation, with abelacimab, asundexian, and milvexian undergoing Phase III evaluation. As a once-monthly, fully humanised antibody, abelacimab may improve adherence and eliminate concerns about drug–drug interactions or impaired kidney function in patients with atrial fibrillation at risk of stroke.35
The Burden of Bleeding from the Patient’s Perspective
Mellanie True Hills
Mellanie True Hills, American Foundation for Women’s Health, and StopAfib.org, both based in Greenwood, Texas, USA, began by sharing insights on living with AF, and in particular, the challenges of being on anticoagulation for life. Hills announced that StopAfib.org had recently partnered with the National Blood Clot Alliance (NBCA) to run an online survey supported by Anthos Therapeutics, with the aim of investigating the impact of so-called ‘minor bleeding’ on patients’ quality of life.37 This was defined as bleeding that did not require medical attention, but may nonetheless have felt significant to the person experiencing it. More than 3,000 persons responded to the survey. It was shown that 59% of respondents had experienced bleeding and/or bruising since starting anticoagulation. Of these, almost half reported an emotional impact of these occurrences, ranging from embarrassment to anxiety and depression. In particular, the fear of a future serious bleed, especially if help is not close at hand, was expressed by numerous patients.
More than half of respondents said that they had changed their lifestyle, discontinuing sports and hobbies that carry even a small risk of injury, and limiting household tasks involving knives or sharp objects, including shaving and personal grooming, because they were taking an anticoagulant. Those who continued performing these activities reported having to take extra care, extra time, and sometimes extra expense to protect themselves. Many reported choosing clothing that covers up bruises to avoid the comments or judgement of others. In many cases, issues that may seem trivial to healthcare professionals emerged as major preoccupations for patients.
A further notable finding was that the impact on emotions and lifestyle was greater for younger people (aged 21–49 years; n=408) compared with older people (aged 50 years or over; n=1,407). Some younger respondents reported feeling socially isolated, as their peers could not meaningfully relate to their health issues. In fact, of all bleeding incidents, the greatest emotional impact came from heavy periods in premenopausal females, who were forced to stay home from work, and significantly curtail their usual activities while menstruating.
Overall, almost 30% of respondents reported that, as a result of their experiences with bleeding and/or bruising, they had considered pausing their anticoagulant, or actually did pause it. Some (7%) did so without first telling their doctor (Figure 4).37
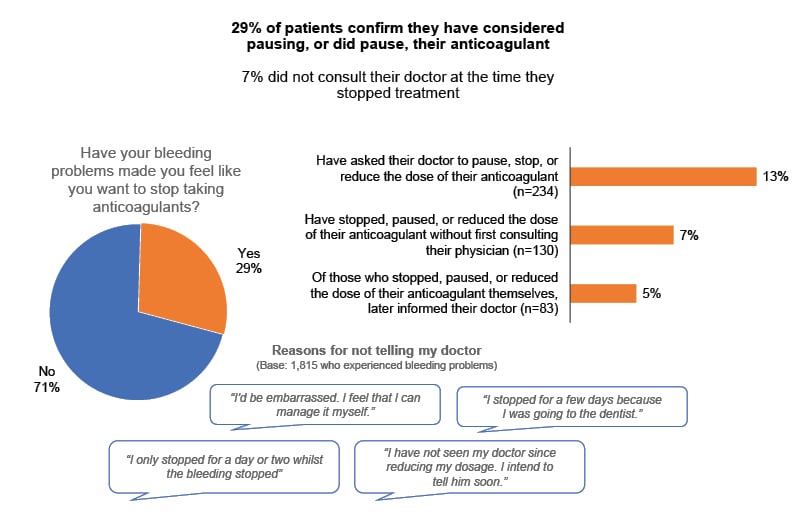
Figure 4: Impact of bleeding problems on patient adherence with their anticoagulant.37
Base: 1,815 (59%) experiencing a bleeding problem on current treatment.
From Patient-Relevant Bleeding Events Among Patients Taking Anticoagulant Medication (Insocius, November 2022): A global survey of 3,000+ patients prescribed anticoagulants, conducted through StopAfib.org and the National Blood Clot Alliance (NBCA), to gain quantitative and qualitative insight on the impact of patient-relevant bleeding events, which were defined as bleeding not requiring medical intervention.37
This finding elevates the problem from a purely quality of life issue to a prognostic one. The bottom line, Hills stated, is that what doctors perceive as ‘minor bleeding’ is anything but to patients. Hills proposed that ‘minor bleeding’ should be reframed as “patient relevant bleeding,” with the recognition that these experiences can seriously jeopardise both quality of life and adherence to anticoagulation regimens. Many patients accept this situation as the cost they have to pay for avoiding strokes and other thromboembolic events, but huge interest was expressed in the possibility of a new anticoagulant with a lower bleeding risk. Thus, Hills concluded: “We hope that the emerging class of Factor XI inhibitors will be the answer for us.”







