Chairperson: Aristoteles Giagounides1
Speakers: Aristoteles Giagounides,1 John Grainger,2 Drew Provan,3 Maria L. Lozano,4
1. Marien Hospital Düsseldorf GmbH, Düsseldorf, Germany
2. Royal Manchester Children’s Hospital, Manchester, UK
3. Barts and The London School of Medicine and Dentistry, London, UK
4. Hospital JM Morales Meseguer, University of Murcia, Murcia, Spain
Disclosure: Prof Giagounides has received fees for advisory board attendance from Amgen and Novartis; and is a Data Monitoring Committee member for Amgen. Prof Grainger has received funding from Alexion, Amgen, Dova Pharmaceuticals, Inc., GlaxoSmithKline, Novartis, and Ono Pharmaceutical Co., Ltd. Prof Provan has received research support from Amgen and Novartis; speaker honoraria from Amgen and Novartis; and consultancy fees from MedImmune, LLC, Ono Pharmaceutical Co., Ltd., and UCB. Prof Lozano has received grants and research support from Amgen and Terumo Medical Corporation; and has received honoraria and consultation fees from Amgen, Alexion, Celgene, GlaxoSmithKline, Grifols, Novartis, and Sobi.
Acknowledgements: Medical writing assistance was provided by Charlotte Watson, Elements Communications Ltd., Westerham, UK.
Support: This report summarises presentations given by the speakers at an Amgen Europe-sponsored satellite symposium held at the 25th EHA Annual Congress, on 11th June 2020. The publication of this article was funded by Amgen. The views and opinions reflect those expressed by the speakers and are not necessarily those of Amgen.
Citation: EMJ Hematol. 2020;8[1]:30-38.
Meeting Summary:
The latest developments in treatment recommendations and new management strategies for patients with immune thrombocytopenia (ITP) were highlighted at this symposium. There is a renewed focus on improving not only clinical outcomes such as bleeding, but also quality of life (QoL) for patients with ITP, as well as increased recognition of the importance of patient involvement in their treatment decision making. Thanks to an expanding body of clinical data and better understanding of the underlying disease pathogenesis, the concept of tailoring ITP treatment to individual patients is becoming a reality. Therapies such as thrombopoietin receptor agonists (TPO-RA) play an important role in this transformation of patient management. TPO-RA are recommended for use in ITP based on robust clinical evidence, and during the COVID-19 pandemic their importance as nonimmunosuppressive therapies has been highlighted in several guidelines. Generally in newly diagnosed patients with ITP, nonimmunosuppressive therapies such as intravenous Ig (IVIG) and TPO-RA are recommended over corticosteroids,1–4 and for patients with chronic ITP it is suggested to not make any alterations to therapy.1,2
Some TPO-RA already have a range of licensed applications outside of ITP, such as myelodysplastic syndrome (MDS) and chemically induced thrombocytopenia. Their full potential is still under investigation and promising preliminary clinical studies have indicated that TPO-RA may have therapeutic applications in other diseases.
Using ASH Guidelines and ICR Recommendations to Guide Clinical Decisions
Professor John Grainger
The American Society of Hematology (ASH) guidelines and the International Consensus Report (ICR) recommendations were first published in 20105 and 2011,6 with updates presented in December7 and November 2019,8 respectively. In this period, new clinical evidence and treatments for ITP became available and both the ASH and ICR recommendations have incorporated these updates to present a comprehensive framework for ITP patient management.
The importance of QoL for patients and individualisation of treatment via patient involvement in decision making is given new weight in the updated publications. In the ICR recommendations, treatment goals are individualised to the patient and phase of disease and the maximisation of QoL features is the treatment aim, alongside prevention of severe bleeding, providing safe platelet levels, and minimising toxicity.8 In the ASH guidelines, patient values and preferences are considered when selecting second-line therapies.7
In both updates there is now an emphasis on moving away from immunosuppressive therapies. Corticosteroids remain the standard first-line therapy, but their use in short courses (<6 weeks) only is recommended.7,8 TPO-RA are not considered as initial therapies in either the ASH guidelines or the ICR recommendations,7,8 and rituximab is only suggested as an adjunct to corticosteroids in specific cases in the ASH guidelines.7
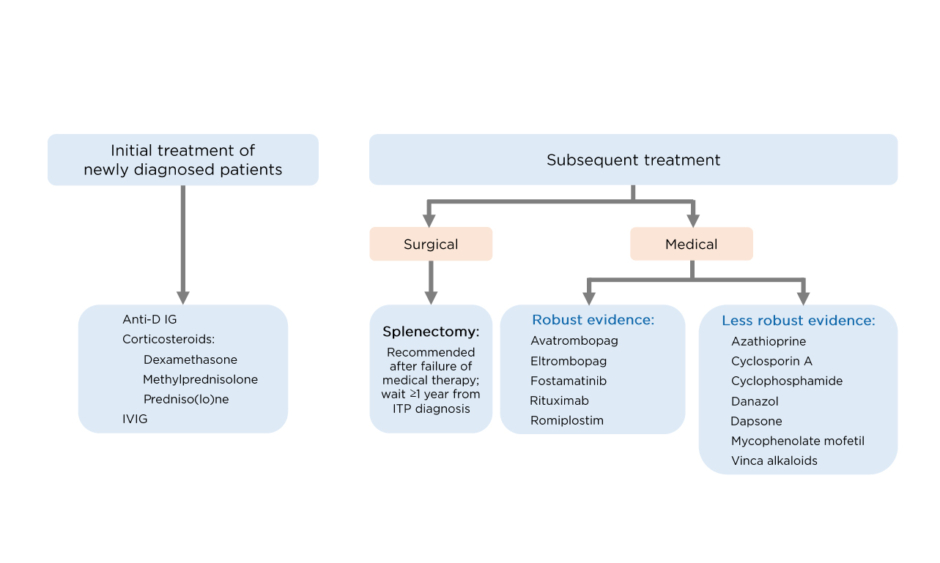
With respect to second-line therapies in adults, both the ICR recommendations and ASH guidelines advocate the earlier use of TPO-RA (as soon as 3 months after diagnosis).7,8 The ICR recommendations indicate that there is robust evidence for the use of TPO-RA, fostamatinib,9 and rituximab as second-line therapies (Figure 1). These should be used in preference to splenectomy, which should generally be deferred for adults who have been diagnosed with ITP for >1 year.8 In the ASH guidelines, the recommendation for adults with ITP of >3 months is to use either a TPO-RA (romiplostim or eltrombopag) or rituximab, with splenectomy suggested as an option after 12 months. Rituximab is preferred over splenectomy and TPO-RA over rituximab, although the ASH guidelines focus on patient values in this setting and defer to patient preference for which therapy is given.7
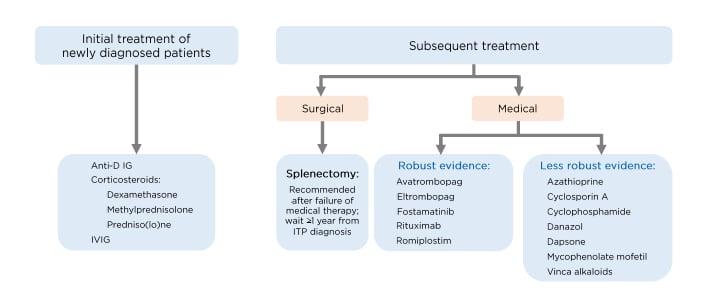
Figure 1: Treatment of immune thrombocytopenia in adults according to the International Consensus Report (ICR) recommendations.
IVIG: Intravenous Ig; ITP: immune thrombocytopenia.
Adapted from Provan D et al.5
For paediatric patients with ITP, both the ICR recommendations and ASH guidelines focus on bleeding scores for guiding treatment decisions and suggest observation of newly diagnosed patients who have no or mild bleeding. In more severe cases of bleeding, short courses of corticosteroids are recommended as initial treatment.7,8 The ICR also recommends IVIG if a rapid increase in platelet count is required,8 but the ASH guidelines take a more conservative approach to the use of IVIG in this setting, recommending preferential use of corticosteroids.7
With respect to persistent or chronic ITP, the ICR recommendations note that paediatric patients with stable platelet counts can be managed with observation, corticosteroids, and IVIG as rescue therapy for acute bleeding. TPO-RA are preferred in cases of persistently low platelet counts based on the number of clinical studies demonstrating good responses, reduction in bleeding frequency, improved QoL, and a good safety profile. In cases of lack of response to first-line therapy in paediatric patients, the ASH guidelines recommend TPO-RA in preference to either splenectomy or rituximab, but acknowledge the importance of patient preference in the overall selection of therapy.7 In those who fail to respond to TPO-RA, especially adolescent females, the ICR recommendations suggest rituximab plus dexamethasone as an alternative option.
If response to a TPO-RA is lost, adding mycophenolate mofetil or an alternative immunosuppressant, or switching to another TPO-RA, may be considered.8
Thrombopoietin Receptor Agonists in the New ICR Recommendations: Improving Treatment Strategies
Professor Drew Provan
In the 10 years since the first ICR recommendations were published, the use of TPO-RA in clinical practice has dramatically increased and they are now widely used to treat patients with ITP. The updated ICR recommendations reflect this increase in use and provide detail on the most appropriate use of TPO-RA in clinical practice.
Adult Patients
Whilst the approved starting dose of romiplostim is 1 µg/kg per week,10 the average weekly romiplostim dose in most studies is 3–5 µg/kg, and more than one study has used 3 µg/kg as the starting dose.11-13 Platelet responses are typically seen within 2 weeks,14,15 and lengthened dose intervals for romiplostim have not been shown to be effective.16 The approved dose of eltrombopag is 25–75 mg/day, with the daily dose decreased for platelet counts >200×109/L.17,18 TPO-RA provide excellent responses in both splenectomised and nonsplenectomised patients; in an integrated analysis of romiplostim in 1,111 patients with ITP, platelet responses were seen in 82% and 91% of patients with and without splenectomy, respectively.19 Responses have been monitored in clinical studies for up to 6–8 years, often allowing other ITP therapies to be reduced or discontinued. A good example of this is seen in the EXTEND study, an open-label extension study for patients with ITP receiving eltrombopag. Platelet counts increased rapidly over the first 2 weeks of treatment and were maintained over the duration of the study (250 weeks).20,21
A patient starting TPO-RA therapy often asks whether the treatment will be lifelong. Although cessation of TPO-RA treatment leads to a return of thrombocytopenia in many cases, some patients may achieve a durable response after withdrawal of a TPO-RA. In a Phase II single-arm study, remission was demonstrated in 32% of patients with ITP who received romiplostim within 6 months of diagnosis.11 In another study, 28% of patients with chronic ITP who received romiplostim for >6 months achieved remission.22 Treatment-free remission has also been seen with eltrombopag; in one retrospective study, 53% of evaluable patients with ITP who responded to eltrombopag sustained a response for ≥6 months after discontinuation.23
Failing multiple therapies is a common problem that worries clinicians and patients. This issue is addressed in the ICR recommendations. If a patient fails to respond to a TPO-RA, switching from one TPO-RA to another can be considered; this has shown a positive effect on response and tolerability.24-27 One study has shown switching TPO-RA to be effective in 50–80% of patients, with eradication of platelet fluctuations in 54% of patients and resolution of adverse events in all patients.24 The ICR also noted that the addition of small doses of corticosteroids to prior lines of therapy should be explored in patients who have failed multiple therapies.8
Emergency treatment is required when patients sustain severe bleeding or to reduce the risk of sudden bleeding. According to the ICR recommendations, a combination of treatments, including IV corticosteroids and IVIG, should be used in emergency situations. Platelet transfusions may also be helpful. In the case of life-threatening bleeding and the absence of a significant response to the above options, TPO-RA may be considered.8
Paediatric Patients
TPO-RA are recommended in chronic or persistent ITP in children, and this recommendation is supported by multiple clinical studies. In a key Phase III randomised, double-blind study of romiplostim in children, a durable platelet response of 52% and an overall response of 71% was demonstrated.28 In the paediatric ITP setting, these figures are good. As for adult patients, switching TPO-RA is also recommended if response to a TPO-RA is lost.8
Tailoring Immune Thrombocytopenia Treatment to the Individual
Professor Maria L. Lozano
Personalising management for patients with ITP is important, although there are challenges in optimising therapeutic strategies. For patients who receive treatment for their ITP, despite diverse pathophysiological differences, treatment strategies are often very similar. Consequently, some patients respond better to treatment than others. In an ideal world, in order to better tailor ITP treatment to the individual, management should be differentiated based on underlying disease mechanism and biological characteristics (Figure 2).
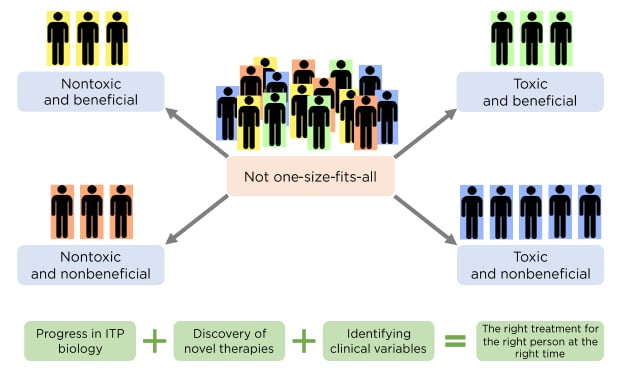
Figure 2: Principles behind tailoring treatment to individual patients.
ITP: immune thrombocytopenia.
Courtesy of Prof Lozano.
New clinical data in first-line therapy of ITP could potentially guide and tailor treatment decisions. According to current guidelines, corticosteroids are the mainstay of initial ITP treatment,7,8 but which newly diagnosed patients require steroid treatment and which steroid and dose to use are both important questions. The consensus is to initiate therapy in patients who experience bleeding or have a low platelet count, but a recent publication sought to provide granularity as to who is most at risk of bleeding and, therefore, would most benefit from corticosteroid treatment. The relationship between different factors and haemorrhagic manifestations was studied in 20,000 adult patients with a new diagnosis of ITP in a Japanese registry. Results indicated that previous haematuria, platelet count <15×109/L, and age >60 years were relevant contributors to an increased risk of bleeding.29 In terms of which corticosteroid to initiate, a meta-analysis has shown that dexamethasone works faster than prednisolone in terms of complete response at 14 days, and with a similar safety profile.30 Because sustained remission rates were similar at 6 months, either agent was deemed appropriate, but, in the absence of contraindications, responses to dexamethasone may be more rapid. With respect to steroid dosing, for elderly patients or those with underlying diseases and a high or higher probability of adverse events, doses of <1 mg/kg of prednisolone may be more suitable.
Since patients with ITP are at increased risk of thrombosis, and some ITP therapies contribute to this risk, it would be valuable to be able to identify patients who may develop this complication.31,32 A retrospective study of 7,225 patients with ITP in France identified several risk factors for arterial thrombosis and venous events in patients undergoing splenectomy. Age, male sex, previous arterial thrombosis, and exposure to antiplatelet drugs were associated with arterial thrombosis, and age, cancer, and a history of venous thromboembolism were related to venous events.31
Regarding appropriate selection of second-line therapies, a recently published retrospective analysis evaluated the use of TPO-RA in adults with ITP. This found that, in clinical practice, severity of ITP is a key factor in the choice of TPO-RA. At the time of ITP diagnosis and on the day of TPO-RA initiation, bleeding symptoms were more frequent and more severe, and platelet counts were significantly lower among patients who were assigned to romiplostim.33 With respect to rituximab, small retrospective studies have indicated that females in the early phases of ITP (rituximab plus dexamethasone)34 or >40 years of age35 seem to have the highest probability of sustained response. In a recently published study assessing the long-term efficacy and safety of rituximab,36 again, women >40 years of age seemed to have the best response. However, there were no significant differences in response with respect to sex. Considering splenectomy, and whether surgery or medical therapy is most appropriate for long-term management of ITP, it is important to discuss the risks and benefits of splenectomy with patients, and to actively encourage their participation in treatment decisions.
There is currently no single approach for the management of multirefractory patients. The best management approach may not be to administer single agents in a sequential manner, but rather to consider combination therapy, targeting multiple pathways by which thrombocytopenia can occur. In addition, recent incorporation of new treatments such as TPO-RA mean that some patients with chronic ITP may now be able to achieve treatment-free responses (TFR). In a retrospective study, 51% of patients who received romiplostim without subsequent TPO-RA switching were able to sustain a TFR upon discontinuation of therapy.
The number of patients achieving TFR with eltrombopag (24%) appeared to plateau after the first year, but the number of patients achieving TFR progressively increased over time in the case of romiplostim. These results still need to be validated but are an indication that a high number of patients may be able to achieve TFR with medical therapy alone (Figure 3).33 Prof Lozano also noted that high response rates and good safety profiles of TPO-RA make these agents especially beneficial for elderly patients with ITP, who experience a high frequency of serious bleeding and an inherently high thrombotic risk.
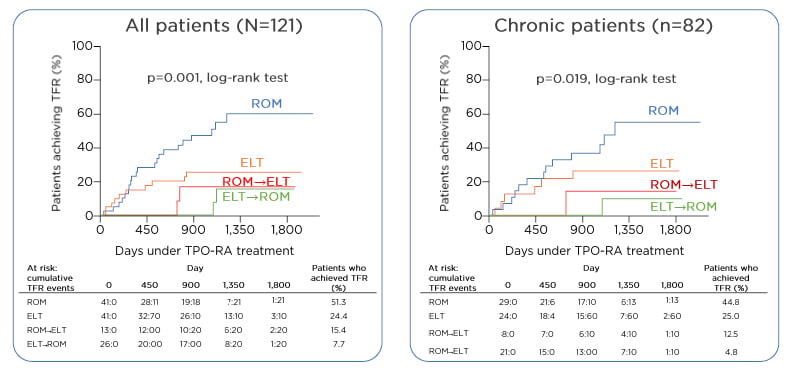
Figure 3: Treatment-free response in immune thrombocytopenia with thrombopoietin receptor agonists.
ELT: eltrombopag; ROM: romiplostim; TFR: treatment-free response; TPO-RA: thrombopoietin receptor agonist.
Adapted from Lozano ML et al.33
Thrombopoietin Receptor Agonists Beyond Immune Thrombocytopenia
Professor Aristoteles Giagounidis
TPO-RA have been investigated in several settings outside of ITP and chemotherapy-induced thrombocytopenia, for example in aplastic anaemia (AA) and MDS.
Aplastic Anaemia
At presentation, virtually all patients with AA have severe thrombocytopenia. The consequences of this can be severe or even life-threatening, for example in cases of intracranial haemorrhage. Current treatment typically consists of platelet transfusions whilst awaiting a response to immunosuppression or haematopoietic stem cell transplantation. However, TPO levels are typically elevated in AA, suggesting a therapeutic role for TPO-RA in this setting.37-39 Clinical studies with TPO-RA in patients with AA have indicated that both romiplostim and eltrombopag can improve haematological responses in this setting. A Phase II/III study was undertaken to investigate romiplostim in patients with AA who were ineligible or refractory to immunosuppressive therapy. A haematological response was seen in 84% of patients at 27 weeks, trilineage responses were seen in 26% of patients, and the median time to any haematological response was 37 days.40 Another study with romiplostim in 35 patients with AA investigated platelet responses over a longer period. Platelet response rate was 36% at Week 27, which was well maintained up to Week 105 (30%).41 Eltrombopag was also investigated in a seminal study of 43 patients with AA; at 12–16 weeks, 40% of patients had (any) haematological response and at the last follow-up (median 12 months), 16% had a trilineage response.42,43
Myelodysplastic Syndrome
Despite the incidence of severe thrombocytopenia in MDS only being 7–19%, it is still of concern because it indicates poor prognosis. Bleeding is also the second most common cause of death in MDS after sepsis.44-48 Management of thrombocytopenia in patients with MDS may include platelet transfusions, IVIG, steroids, and disease-modifying agents such as azacitidine or decitabine.49-51 TPO is elevated in MDS, again suggesting the potential for TPO-RA as therapy in this setting. In several Phase II studies, TPO-RA were found to have a positive effect on platelet counts and, in some cases, reduced the requirement for platelet transfusions. Romiplostim has been studied in patients with lower-risk MDS in a Phase II placebo-controlled study: in patients with platelet levels ≥20–50×109/L, the number of bleeding events was significantly reduced (p<0.0001), and in patients with platelet levels <20×109/L, the number of platelet transfusions was significantly reduced compared with placebo (p<0.0001). However, there was no difference in overall survival.52 The Phase II EUROPE study investigated endogenous TPO levels and their predictive power in patients with low-risk MDS receiving romiplostim. Response rates were higher in patients with lower TPO levels and in patients who had pretreatment transfusion needs of <6 units in the past year. However, despite romiplostim being more effective in this subgroup of patients, neither platelet transfusion requirement nor TPO levels were significantly associated with response.53 Eltrombopag has been investigated in 90 patients with low-risk MDS, low platelet counts, and receiving supportive care. Eltrombopag had a significantly increased response rate versus placebo (47% versus 3%; p=0.0017).54
Conclusion
Over the last few years, the development of targeted therapies and emergence of new clinical data have enhanced outcomes for patients with ITP. This has opened the door to the possibility of individualisation of treatment, a concept now recognised in the updated ICR recommendations and ASH guidelines. Individualisation of treatment and greater consideration for patients’ QoL are the cornerstones of contemporary management strategies in ITP. TPO-RA play an important role in this new management paradigm, as supported by the updated ICR recommendations and ASH guidelines. Mutual participation in treatment decisions between patients and physicians is also recognised, and this collaborative approach to treatment should be integral when putting these new recommendations into clinical practice. TPO-RA have already been demonstrated to have promising applications outside of ITP and have even further potential in the treatment of other diseases in the future.