Introduction from the Chair
Prof Sehn opened the symposium with an overview of diffuse large B-cell lymphoma (DLBCL) focussing on key milestones in treatment developments. Rituximab (R), a CD20-directed monoclonal antibody (mAb), in combination with the chemotherapy cocktail CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) was the first major improvement in lymphoma over chemotherapy. R-CHOP was later approved as first-line management of DLBCL, demonstrating a marked improvement in overall survival (OS).1,2
Prof Sehn stressed that there remain unmet needs in DLBCL treatment, especially among relapsed and refractory (R/R) DLBCL patients.
Click here to view Prof Sehn’s INTRODUCTION
Diffuse Large B-Cell Lymphoma: What Options do Patients Have for First-Line Treatment?
Doctor Armando López-Guillermo
Dr López-Guillermo introduced DLBCL as the most common aggressive non-Hodgkin lymphoma (NHL) subtype in the Western world, constituting 30–58% of all lymphomas.3-7
DLBCL was considered an incurable disease until the advent of polychemotherapy (e.g., CHOP) in the 1970s, rendering DLBCL a curable disease for some patients.8-10 In recent decades, treatment options have evolved significantly; in 1997, R became the first mAb approved for the treatment of NHL.11 In DLBCL, R-CHOP demonstrated substantial improvements in OS compared with CHOP alone,12 becoming the gold standard for these patients.
Despite improved OS provided by R-CHOP, one-third of patients remain either refractory to initial therapy or relapse after treatment, and their prognosis remains poor.13
Heterogeneity of Subtypes
DLBCL is a heterogeneous disease comprising several distinct subgroups, with varied prognoses and clinical outcomes. Several new subcategories of lymphomas have been recognised by the World Health Organization (WHO) in the 2016 classifications, including the cell of origin (COO) germinal centre B-cell-like (GCB) and activated B-cell-like (ABC) subgroups, and the issue of MYC, BCL2, and BCL6 alterations. A new category, the ‘high-grade B-cell lymphoma’ (with and without MYC and BCL2 or BCL6 translocations), has emerged.14,15
Dr López-Guillermo emphasised that characterisation and risk stratification of DLBCL subgroups is an evolving process and ongoing efforts to tailor therapy based on the different oncogenic pathways require COO classification.14-20 Although gene expression profiling is not routinely available, surrogate algorithms based on immunohistochemistry or quantification of RNA transcripts provide concordant results and are acceptable.21 Most studies have reported poorer outcomes among ABC-type DLBCL patients.14,22
The WHO 2016 update also recognised the rearrangement of MYC and BCL2 and/or BCL6 (referred to as ‘double-hits’ or ‘triple-hits’ [DT/TH]) as new adverse prognostic markers, as well as their immunohistochemical co-expression of MYC and BCL2 and/or BCL6 proteins (defined as ‘double-expressors’ [DE]). Expression does not equate to rearrangement; DE are not the same as DH/TH.15,23 Patients with high-grade B-cell lymphoma have poor outcomes and tend to be older, present with advanced stage, have high-risk International Prognostic Index (IPI), and exhibit central nervous system involvement. DH, which constitute 5–10% of DLBCL, are of germinal centre B-cell type.14-16,19,22
CAN WE IMPROVE ON R-CHOP?
In the last decade, multiple efforts have been made to improve the clinical outcomes of DLBCL, primarily focussing on increasing the intensity and density of current therapies, addressing maintenance, and introducing novel antibody therapies, immunomodulatory drugs, and small molecules in combination with R-CHOP.
Intensification of Chemotherapy
Attempts to improve the efficacy of first-line therapy through dose-dense and dose-intensified regimens, the use of different induction schedules, or the early intensification of R administration have not demonstrated any survival advantage as compared with standard R-CHOP.
The only positive trial published comparing dose-intensive R-ACVBP (R, doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone) with subsequent sequential consolidation versus R-CHOP reported superior progression-free survival (PFS) and OS in the intensified R-ACVBP group in young patients with only one adverse prognostic factor, as defined by the age-adjusted IPI.24
Two Phase III trials comparing dose intensification with 14-day versus 21-day cycles25 concluded that R-CHOP-14 was not superior to R-CHOP-21; there was no survival advantage of R-CHOP-14 over R-CHOP-21. Furthermore, no molecular or clinical subgroup benefitted from dose intensification in the study.25,26
Regarding infusional regimens, Bartlett et al.,27 showed that although some subset differences are seen with DA-EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and R) versus R-CHOP, OS remains consistent across the full population.
Finally, several randomised control trials (RCT) evaluating the role of up-front autologous stem-cell transplantation (ASCT) following R-CHOP (as consolidation treatment) have not validated an OS benefit for these patients.4,24,28-30
Maintenance
Dr López-Guillermo confirmed that maintenance does not play a role in the first-line therapy of DLBCL; however, Thieblemont et al.,31 recently demonstrated significantly prolonged PFS (but not OS) in a group of elderly DLBCL patients randomly assigned lenalidomide as maintenance therapy versus placebo following complete or partial response (CR/PR) to R-CHOP.
Antibodies
Results of the GOYA trial indicated that replacement of R-CHOP with obinutuzumab (a glycoengineered, Type II, anti-CD20 mAb) in combination with CHOP did not improve PFS or OS in frontline treatment of DLBCL.2
Immunomodulatory drugs and small molecules with targeted action
Several recent randomised trials mainly targeting ABC phenotypes are investigating the survival benefit of immunomodulatory drugs (e.g., IMiD) or other small molecules added to standard R-CHOP. No survival benefit with bortezomib-R-CHOP, ibrutinib-R-CHOP, or lenalidomide-R-CHOP versus R-CHOP in front-line DLBCL has been observed.32-35
FIRST-LINE DIFFUSE LARGE B-CELL LYMPHOMA: WHERE DO WE STAND NOW?
Dr López-Guillermo reiterated that there remains a group of patients with poor outcomes despite treatment with R-CHOP. Several targeted therapies and small molecules have been evaluated to improve R-CHOP; however, the main challenges remain to improve CR rates and to avoid relapse.
Click here to view Dr López-Guillermo’s presentation
What is Next for Patients who Relapse or are Refractory to Treatment?
Professor Matthew Matasar
Prof Matasar opened by reiterating the persistent unmet needs of R/R DLBCL patients.36 Approximately 50% of R/R DLBCL patients are eligible for ASCT, and only half of those will be cured by transplant. Patients who are transplant-ineligible or who relapse following transplant have few treatment options, with survival measured in terms of months.37
Response rates subsequent to salvage therapy remain low in patients with R/R DLBCL, as highlighted by the SCHOLAR-1 data. For patients with refractory DLBCL, the objective response rate was 26% (CR: 7%) to the next line of therapy.38
Transplant-Eligible Patients
Platinum-based salvage therapy prior to transplant remains the standard of care for transplant-eligible patients; however, there is no one universally accepted or preferred (combination) platinum-based programme. Several different salvage regimens have been evaluated in several trials, including standard R-DHAP (dexamethasone, cytarabine, cisplatin) versus R-ICE (ifosfamide, carboplatin, etoposide), R-GDP (gemcitabine, dexamethasone, cisplatin), or O (ofatumumab)-DHAP, all of which have demonstrated poor outcomes.36,39-41
Prof Matasar noted that cross-study comparisons are difficult because of the heterogeneity of studies as well as the variability in inclusion criteria and evolution of response criteria over time.42
There is a lack of real-world data to describe treatment patterns in R/R patients. Herrera et al.43 looked at practice patterns in the US community setting and reported a wide variance in treatment regimes. Approximately one-third of patients received R-ICE at some point in their relapse course; 20% of patients received R-GemOx (gemcitabine + oxaliplatin), and BR (bendamustine + R) was administered to approximately 10% of the population studied.43
Although bendamustine is not a U.S. Food and Drug Administration (FDA)-approved treatment for DLBCL in any line of therapy, as monotherapy it has shown encouraging efficacy with relatively mild toxicity in R/R DLBCL and is frequently used as one of several regimens in this position. Using real-world data, Ionescu-Ittu et al.44 retrospectively compared BR to R-GemOx in transplant-ineligible R/R DLBCL patients, reporting a median OS of 11–13 months, and comparable OS rates for BR and R-GemOx, respectively.
Targeted Therapies in Relapsed/Refractory Patients
There is a paucity of published data around the efficacy of small molecules in R/R DLBCL. Preliminary analysis of a Phase III study comparing lenalidomide monotherapy with the investigator’s choice of monotherapy (e.g., gemcitabine, R, etoposide, or oxaliplatin) suggests similar OS, with lenalidomide showing benefits largely limited to ABC-type DLBCL.45
Ibrutinib has also shown efficacy in patients with ABC subtypes, especially among those exhibiting DE (overall response rate [ORR]: 47%; CR: 37%);46 however, response to ibrutinib monotherapy tends to be brief, with median PFS and OS of 5.5 months and 8.2 months, respectively.46 Prof Matasar was of the opinion that while ibrutinib has some activity, perhaps monotherapy may not be the best way of administering it.
Chimeric antigen receptor T-cells (CAR-T) are emerging as a novel treatment modality; they have demonstrated activity, inducing durable CR lasting >2 years in some patients.
Two ongoing multicentre trials, ZUMA-1 and JULIET, have recently published safety and efficacy of two anti-CD19 CAR-T (ZUMA-1: axicabtagene ciloleucel; JULIET: tisagenlecleucel) in patients with refractory DLBCL.47,48 The median follow-up time in ZUMA-1 and JULIET was 27.1 months and 14.1 months, respectively. The results in both trials were significant with regard to the primary endpoint. ZUMA-1 reported best ORR of 82% (CR: 54%), a 12-month PFS and OS of 44% and 59%, respectively; likewise, JULIET reported ORR of 52% (CR: 40%), 12-month PFS and OS rates of 83% and 49%, respectively. Results were not without CAR-T-related toxicity: between 11% and 22% of patients experienced a Grade ≥3 cytokine release syndrome; between 12% and 32% experienced a Grade ≥3 neurological adverse event (AE); and between 48% and 58% of patients experienced serious Grade ≥3 AE.47,48 ZUMA-7 is an ongoing trial that will help determine which line of therapy CAR-T cells are best suited.49
Click here to view Prof Matasar’s presentation
Can Novel Antibody Therapies Improve Outcomes for Patients with Relapsed/Refractory Diffuse Large B-Cell Lymphoma?
Professor Franck Morschhauser
Immunotherapy is an evolving modality and tremendous advances have been made in targeting the lymphoma microenvironment. Prof Morschhauser began the session by presenting an overview of immune targets and various immunotherapy agents currently under investigation in DLBCL. B-cell malignancies present alternative targets beyond CD20: B cells typically express a variety of antigens including CD19, CD20, CD22, CD30, CD79a, CD79b, and CD45. These targets can be exploited to target B-cell cancers (Figure 1).50-63
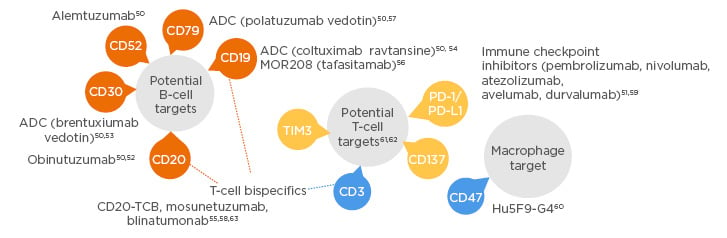
Figure 1: Novel antibody therapies under investigation for diffuse large B-cell lymphoma.
ADC: antibody–drug conjugate.
The focus of Dr Morschhauser’s presentation was to highlight and discuss some of the more significant advances recently made in the antibody field; potential B-cell targets include anti-CD30, anti-CD79, and anti-CD19, whereas potential T-cell targets being explored are PD-1/PD-L1 immune checkpoint inhibitors, T-cell Ig and mucin-domain containing-3 (TIM-3), anti-CD3 or anti-CD137, and T-cell bispecific antibodies. Targeting macrophages is also being investigated (anti-CD47).
Potential T-Cell Targets: Anti-PD-1/PD-L1
Nivolumab and pembrolizumab are two PD-1-targeted T-cell checkpoint inhibitory antibodies (humanised IgG4 anti-PD-1 mAb) with promising activity. As a single agent, nivolumab had a favourable safety profile (AE rate: <1%), but with low ORR among patients ineligible for transplant (ORR: 3%) or those who had failed transplant (ORR: 10%).64 Likewise, pembrolizumab has demonstrated low rates of toxicity but with more encouraging response rates (ORR: 45%; CR: 13%) in R/R PMBC patients.65
Potential Macrophage Targets: Anti-CD47
CD47 is an antiphagocytic signal that is overexpressed to enable the immune evasion of macrophages and other phagocytes. The Hu5F9-G4 antibody is a macrophage immune checkpoint inhibitor which blocks CD47 and selectively eliminates malignant cells while sparing healthy ones. In combination with R, Hu5F9-G4 enhances macrophage mediated antibody-dependent cellular phagocytosis. In a Phase Ib study, heavily pretreated and refractory to the most recent regimen participants were administered Hu5F9-G4 + R. The combination therapy showed promising activity with ORR of 40% and CR of 33%.60
Potential B-Cell Targets: Anti-CD19
CD19 is broadly expressed across the B-lymphocyte lineage and enhances B-cell receptor signalling. In a recent Phase IIa study, the anti-CD19 drug tafasitamab had clinical activity as a single agent in R/R DLBCL. The ORR was 26% and CR rate was 6% in R/R DLBCL patients with a median of two prior therapies, of whom 69% were refractory to R and 74% had relapsed within 12 months of their most recent treatment. A response of 26% is not satisfactory, calling for further combination therapy trials.66
The combination of tafasitamab and lenalidomide is currently being investigated in the Phase II L-MIND study. Based on investigator assessments, tafasitamab in combination with lenalidomide has demonstrated encouraging activity. The median PFS was 12.1 months with a median follow-up time of 17.3 months.67-69
Bispecific Antibodies
Bispecific antibodies (BsAb) refer to antibodies that can bind two different antigens. If these antigens are on B cells and T cells, they can trigger the formation of an immune synapse.70-72 There are many different BsAb constructs with different properties.
Blinatumomab is a bispecific construct derived from the variable fragments of two distinct parental murine mAb, binding both CD19 and CD3. In a recent Phase II study, blinatumomab demonstrated moderate efficacy, with an ORR of 37%. This therapy is administered by continuous intravenous infusion for a single 70-day Cycle 1 and an optional 28-day Cycle 2. Results identified 24% of patients with Grade 4 AE and 17% of patients with AE leading to treatment discontinuation.73
Mosunetuzumab is a full-length, fully humanised BsAb targeting both CD3 on the surface of T cells and CD20 on the surface of B cells. In the Phase I study of mosunetuzumab monotherapy in heavily pretreated patients with relapsed NHL, 131 patients (38 with follicular lymphoma [FL], 75 with DLBCL or transformed FL, and 18 with other histology) were considered efficacy-evaluable. Published data from this study showed mosunetuzumab as having a manageable safety profile with most treatment-related AE being transient and reversible. Mosunetuzumab induces durable CR in late-line DLBCL. The best ORR and CR in this group is reported at 34% and 19%, respectively.74
CD20-TCB (RG6026) is a BsAb with a ‘2:1’ format; it possesses two CD20 binding sites and a CD3 binding site, which enable increased tumour antigen avidity, rapid T-cell activation, and enhanced tumour cell killing. The CD20-TCB antibody was administered in a recent multicentre Phase I trial investigating safety.63 Patients received escalating doses of CD20-TCB as intravenous infusions with dose escalation guided by a model implementing the Bayesian continuous reassessment method with overdose control. To reduce the potential risk of cytokine release syndrome, a single dose of 1,000 mg obinutuzumab pretreatment was administered 7 days prior to the start of CD20-TCB. Responses were observed from 15 µg onwards. CD20-TCB induced durable CR in late-line R/R indolent and aggressive B-NHL patients with 57% ORR and 29% CR in the 10 mg cohort. CD20-TCB + obinutuzumab pretreatment displayed promising clinical activity with manageable toxicity.63
Antibody–Drug Conjugates
Antibody–drug conjugates (ADC) are tripartite molecules consisting of a mAb, a covalent linker, and a cytotoxic payload. Many ADC are being investigated for R/R DLBCL in Phase I and II trials.75,76
In a Phase II study, coltuximab ravtansine, which targets CD19, reported a 44% ORR (five complete responses) with Grade <2 haematologic toxicity, ocular disorders, or peripheral neuropathy. However, the activity was not promising in R/R patients, and ORR was <30% with a median PFS of 4.4 months.77
In Prof Morschhauser’s opinion, the two ADC that stand out are brentuximab vedotin (a CD30 target) and polatuzumab vedotin (pola), a CD79b target, both of which have a similar chemistry including a protease-cleavable peptide linker and a monomethyl auristatin E (MMAE) payload.78-81 Brentuximab vedotin is currently indicated for the treatment of adult patients with R/R HL, following ASCT or at least two prior therapies when ASCT or multi-agent chemotherapy is not a treatment option. It is also indicated for the treatment of adult patients with R/R systemic anaplastic large cell lymphoma.
In a recent Phase II study, brentuximab vedotin as single agent or in combination with R in an R/R DLBCL setting was generally well-tolerated and demonstrated similar activity; 44% of patients, regardless of treatment regimen (monotherapy or combination), achieved an ORR of 44% (CR: 17%).82
Click here to view Prof Morschhauser’s presentation
Polatuzumab Vedotin: Clinical Data in Relapsed/Refractory Diffuse Large B-Cell Lymphoma
Professor Laurie H. Sehn
Mechanism of Action
Prof Sehn began by introducing the mechanism of action of pola. CD79b is a B-cell surface antigen present on virtually all mature B cells. Pola is an ADC consisting of an anti-CD79b antibody conjugated to an MMAE payload through a protease-cleavable linker (Figure 2). MMAE is similar in action to vincristine and inhibits B-cell division and growth. Mechanistically, pola exerts its activity by selectively binding to the tumour surface antigen, followed by rapid internalisation and degradation by lysosomes. The release of the cytotoxic MMAE payload then causes a disruption of microtubules leading to cellular apoptosis and cell death. It is believed that after apoptosis, some of the MMAE disseminates into the surrounding microenvironment supporting the tumour cells, suggesting a multi-modal mechanism of action.79,83,84
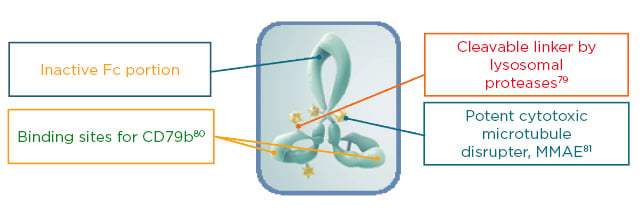
Figure 2: The structure of polatuzumab vedotin: an antibody–drug conjugate targeted to CD79b.
Fc: fragment crystallisable; MMAE: manomethyl auristatin E.
GO29365: A Randomised Phase II Trial of Pola-BR Versus Bendamustine + Rituximab
Study design and preliminary results have been previously described.85,86 Briefly, GO29365 is a global, Phase Ib/II randomised study evaluating the safety, tolerability, and activity of pola in combination with BR in R/R FL or R/R DLBCL.85 Prof Sehn focussed solely on DLBCL results.
In the Phase II randomised portion of the trial, baseline characteristics of patients (n=80) were generally comparable between the arms; the median age of participants ranged from 67 to 71 years, and the majority had advanced-stage disease with a duration of response (DoR) to the last treatment of <12 months in 80% of patients. Around 80% were refractory to their prior therapy and approximately 50% in the pola-BR arm were primary refractory. Patients had received a median of two prior therapies (range: 1–7 prior therapies in the pola-BR arm, and 1–5 in the BR alone arm).85,86
Patients were randomly assigned (1:1) to receive pola-BR or BR for six (21-day) cycles. The results of the primary analysis at the 22.3-month follow-up showed that the pola-BR cohort demonstrated significantly higher ORR (45% versus 18%; p=0.008) and CR (40% versus 18%; p=0.026) compared with the BR alone cohort, regardless of prior treatment status (number of prior line of therapy, refractory, or relapse). Significantly longer DoR (median 10.3 months versus 4.1 months; hazard ratio [HR]: 0.44; p=0.0321) and PFS (median 7.6 months versus 2.0 months; HR: 0.34; p=0.0001) were also seen in the pola-BR cohort compared with BR alone. Furthermore, at the time of analysis, seven patients (18%) in the pola-BR cohort continued to have an ongoing response of >20 months duration. The efficacy of pola-BR was observed regardless of COO or DE status.85,86
Updated clinical data with an additional 6 months follow-up has been pooled together with the six patients from the Phase I safety run-in to look at the trends in long-term survival (median follow-up 27.6 months, maximum follow-up 45.9 months). At 24 months, 31.4% of patients had not progressed; 22.0% of pola-BR patients remain in complete remission at last follow-up (ongoing DoR of >20 months), with only one patient having received subsequent therapy (allogeneic transplant) (Figure 3).87
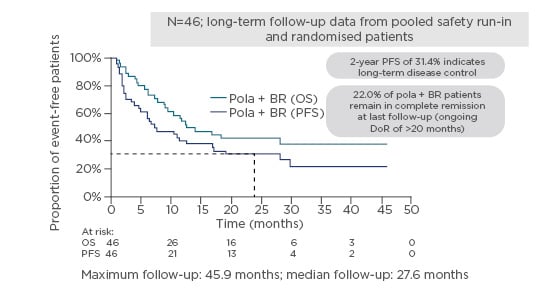
Figure 3: GO29365 – possible long-term survival benefits with pola + bendamustine and rituximab.87
BR: bendamustine and rituximab; DoR: duration of response; OS: overall survival; PFS: progression-free survival.
Pola-BR was well-tolerated; toxicities were generally low grade in nature. Compared with BR, the triple combination demonstrated a slight step-up in toxicity. Infections and cytopaenias were the most commonly Grade 3–4 AE. Pola-BR had higher rates of Grade 3–4 cytopenias (neutropenia, thrombocytopenia,, lymphopaenia, and anaemia) compared with BR, but these did not lead to higher rates of Grade 3–4 infection or result in the need for more transfusions.87
This is the first RCT in this patient population to have demonstrated improvement in survival. Pola-BR recently received FDA approval for the treatment of adult patients with R/R DLBCL, not otherwise specified, after at least two prior therapies, and is awaiting European Medicines Agency (EMA) approval.88
Click here to view Prof Sehn’s presentation
The Future of Relapsed/Refractory Diffuse Large B-Cell Lymphoma Treatment Landscape
Professor Andrew McMillan
Prof McMillan opened with a breakdown of prognoses following outcomes from newly diagnosed DLBCL patients, as well as for those who experience R/R. He concluded that new curative options are needed at all levels and in all settings.
A clear standard of care is warranted in R/R DLBCL patients ineligible for ASCT. In recent years, with remarkable progress in the field of immunotherapy, new therapeutic regimens targeting B cells, T cells, and macrophages, have been developed, and new agents including pola-BR, CAR-T, and bispecifics have entered the therapeutic arena at various settings with promising efficacy.
Pola in Front-Line Therapy
GO29044 was a Phase Ib/II study that evaluated the safety and tolerability of pola in combination with R or obinutuzumab and cyclophosphamide, doxorubicin, and prednisone (CHP) in patients with previously untreated DLBCL and an IPI score of 2–5.89,90 This was an open-label, nonrandomised multicentre study composed of a Phase Ib dose escalation to a maximum pola dose of 1.8 mg/kg, followed by a Phase II dose expansion. Results of this pilot study were recently published by Tilly et al.89 Preliminary clinical activity of pola was promising with an ORR of 89%, CR of 77%, and PR of 12% (median follow-up time of 21.5 months). The safety was as expected and manageable; the most common AE of Grade 3 or worse were neutropenia (30%), febrile neutropenia (18%), and thrombocytopenia (9%).
Pola is currently being investigated in POLARIX,91 an ongoing Phase III placebo-controlled trial with a direct comparison of pola-R-CHP to standard R-CHOP in patients with previously untreated DLBCL and an IPI score of 2–5. The primary endpoint is PFS as assessed by investigator. Secondary outcome measures include event-free survival, CR, and OS.91,92
Pola in Relapsed/Refractory Diffuse Large B-Cell Lymphoma
Prof McMillan discussed the potential role of pola in R/R DLBCL, in particular for patients ineligible for transplant. As Prof Sehn raised, pola-BR represents a clinically meaningful improvement for R/R DLBCL patients where standard treatment options fail. The choice of pola-BR was a pragmatic decision to avoid neurological toxicity from an R-CHOP chemotherapy partner; however, as R-GemOx is frequently used in R/R transplant ineligible populations, a randomised Phase III study comparing pola-R-GemOx to R-GemOx in R/R DLBCL has recently begun.93
Pola will also be investigated in combination with R-ICE in a Phase III study as a bridge to transplant. As ICE is platinum-based, caution is advised due to the risk of neurotoxicity.
Click here to view Prof McMillan’s presentation
Closing Remarks
Professor Laurie H. Sehn
Prof Sehn closed the symposium by summarising the key learnings for both first-line and R/R DLBCL.
- Currently, R-CHOP remains the gold standard in treating first-line DLBCL, but there is still a need to increase the number of patients cured. Targeted therapies (mAb) and small molecules have been investigated, but there has been little improvement to date in treating first-line DLBCL. The POLARIX Phase III study of pola-R-CHP versus R-CHOP may provide an opportunity to improve first-line treatment.
- A variety of anti-CD20/chemotherapy combinations are currently used to treat R/R DLBCL patients, but without strong supporting evidence for any particular one.
- GO29365 has been the first positive RCT supporting pola-BR as a novel therapy for transplant-ineligible R/R DLBCL patients; the recent FDA approval of pola-BR for the treatment of R/R DLBCL after at least two prior therapies was based on the GO29365 results.
- CAR-T are approved in third-line and above DLBCL, but stringent eligibility criteria exist that can make access challenging.







