Abstract
Although hypomethylating agents (HMA) have revolutionised the treatment of myelodysplastic syndromes (MDS), a significant proportion of patients either fail to respond to HMA or their disease progresses after an initial response. Established therapeutic options for these patients remain limited. Fortunately, recent advancements in the knowledge of MDS pathogenesis have allowed for the development of many targeted therapies, including epigenetic regulators, signal transduction regulators, immune checkpoint inhibitors, cell apoptosis regulators, and novel cytotoxic agents. These novel therapeutics have shown varying degrees of promise in clinical trials. Epigenetic regulators, such as second-generation HMA and isocitrate dehydrogenase inhibitors, have shown modest efficacy in early studies, while histone deacetylase inhibitors have, thus far, failed to show significant clinical benefit. Signal transduction modulators, such as transforming growth factor (TGF)-β inhibitors and toll-like receptor inhibitors, appear to alleviate anaemia symptoms, but further studies are needed to determine their effect on survival. Rigosertib, a multikinase inhibitor, improved survival in a small subset of patients with very high-risk MDS. Immune checkpoint inhibitors have shown mixed results. Agents that have recently been approved for use in specific types of high-risk acute myeloid leukaemia, including FMS-like tyrosine receptor kinase 3 inhibitors and CPX-351, are also being studied for use in MDS, with early studies suggesting efficacy. Several other agents are also under investigation with results pending. These novel agents represent potential therapeutic options for patients who have failed HMA and for whom no currently established therapies are available.
INTRODUCTION
Myelodysplastic syndromes (MDS) are a heterogeneous group of disorders resulting from the clonal expansion of a haematopoietic progenitor, leading to bone marrow dysplasia, ineffective haematopoiesis, and increased risk of transformation to acute myeloid leukaemia (AML). Risk factors include older age, male sex, and prior exposure to radiation or chemotherapy.1 Treatment selection is generally based on calculated risk. The International Prognostic Scoring System (IPSS), which stratifies patients with MDS into four risk categories (low, intermediate-1 [INT-1], intermediate-2 [INT-2], and high-risk) based on blast percentage, number of cytopenias, and cytogenetic profile, is the most commonly used risk stratification tool for treatment selection (Table 1). Of note, an updated scoring system, the IPSS-R, is available (Table 2); however, all currently established therapies were approved using the older IPSS system.2 This paper will review both established and emerging therapeutic options for both lower and higher-risk MDS, with an emphasis on novel therapies.
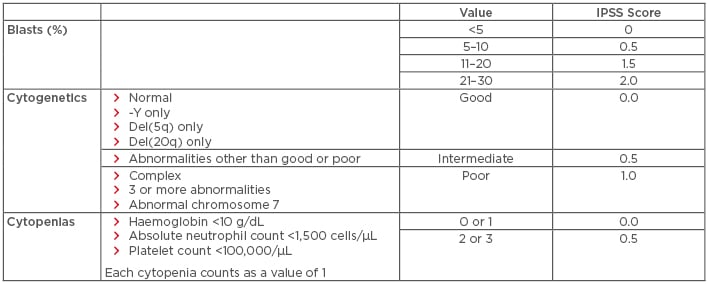
Table 1: International prognostic scoring system for the stratification of myelodysplastic syndromes.
IPSS risk score interpretation: low risk: 0; intermediate-1 risk: 0.5–1.0; intermediate-2 risk: 1.5–2.0; high risk: ≥2.5. Del: deleted; IPSS: International Prognostic Scoring System.
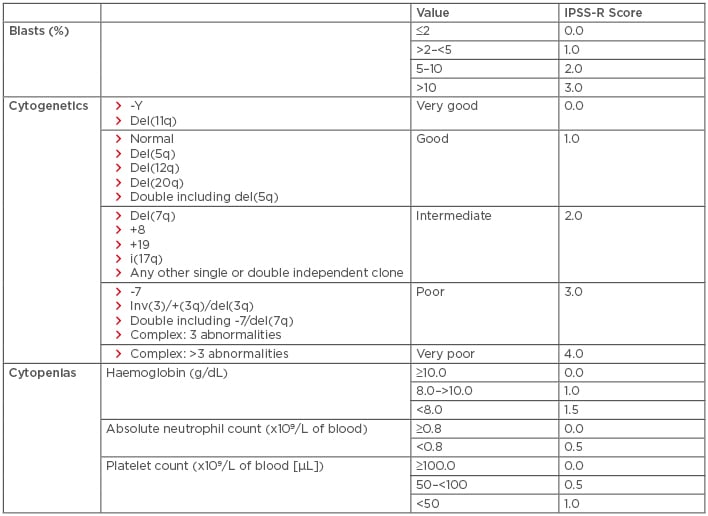
Table 2: Revised international prognostic scoring system for the stratification of myelodysplastic syndromes.
IPSS-R risk score interpretation: very low risk: ≤1.5; low risk: >1.5 to 3.0; intermediate risk: >3.0–4.5; high risk: >4.5–6.0; very high risk: >6.
Del: deleted; IPSS-R: International Prognostic Scoring System-Revised
ESTABLISHED THERAPIES FOR LOWER-RISK MYELODYSPLASTIC SYNDROMES
For patients with lower-risk MDS (INT-1), the decision to treat is often based on the degree of anaemia and transfusion dependence. Patients who are transfusion independent may simply be observed; however, for those who become transfusion dependent, therapy is often warranted. Standard options include erythropoiesis-stimulating agents (ESA), lenalidomide, hypomethylating agents (HMA), and immunosuppressants (Table 3). Of these options, ESA, such as recombinant erythropoietin and darbepoietin, are generally considered first-line therapy in lower-risk MDS, having been shown to alleviate anaemia and possibly overall survival (OS) in this population.3-5 Granulocyte-colony stimulating factor improves the efficacy of ESA through a synergistic effect without increasing the risk of leukaemic transformation; as a result, granulocyte-colony stimulating factor has gained wide clinical acceptance in most countries.5-7 Lenalidomide is considered first-line therapy for those with del(5q), having been shown in clinical trials to produce haematologic improvement (HI) in 56.0% of lower-risk MDS patients, transfusion independence (TI) in 46.0%, and partial or complete cytogenetic response in 71.6%, with best response in those with del(5q).8,9
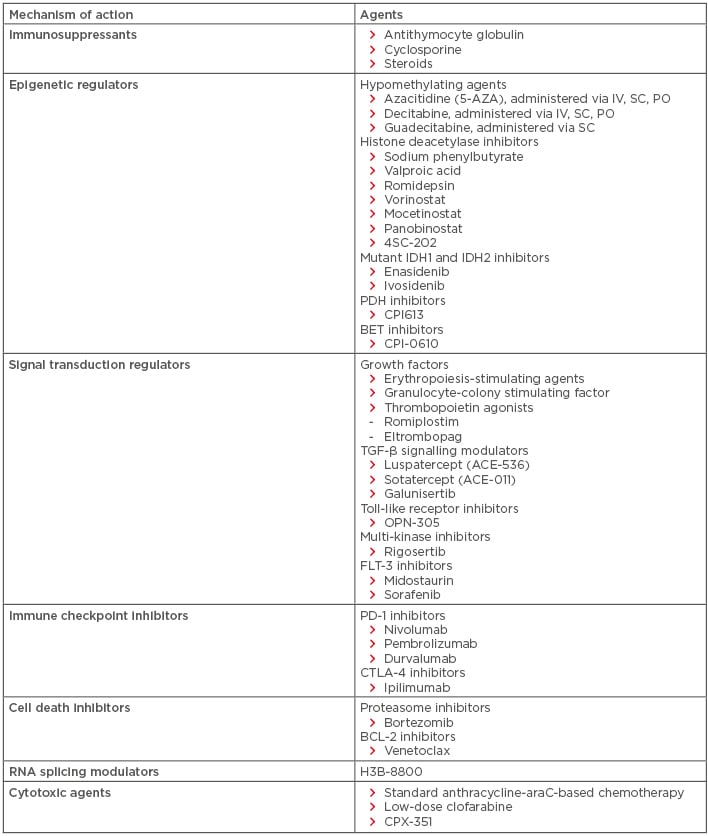
Table 3: Established and emerging therapies for myelodysplastic syndromes.
BCL: B cell lymphoma; BET: bromodomain and extraterminal domain; FLT: FMS-like tyrosine receptor kinase; IDH: isocitrate dehydrogenase; IV: intravenous; PD: programmed cell death protein; PDH: pyruvate dehydrogenase; PO: per os (oral administration); SC: subcutaneous.
HMA have shown efficacy in lower-risk MDS, particularly after ESA and/or lenalidomide failure. In a Phase II clinical trial, low-dose 5-AZA and decitabine showed good overall response rates (ORR) (49% and 70%, respectively), cytogenetic response rates (61% and 25%, respectively), and achievement of TI (32% and 16%, respectively) in this lower-risk population.10 However, further studies are needed to determine whether HMA alter the natural history of lower-risk MDS. Immunosuppressants, including antithymocyte globulin, cyclosporine, and steroids, are also used in lower-risk MDS and have been associated with haematologic response in clinical studies, though no survival benefit has been found (Table 3).11
ESTABLISHED THERAPIES FOR HIGHER-RISK MYELODYSPLASTIC SYNDROMES
For patients with higher-risk MDS (INT-2), HMA are considered the standard of care. 5-AZA has been particularly well-studied in this population by two multicentre randomised trials. In the CALGB 9221 trial,12 191 patients with higher-risk MDS were randomised to 5-AZA or best supportive care. Treatment with 5-AZA was associated with 7% complete response, 16% partial response, longer median time to leukaemic transformation (21 months versus 13 months; p=0.007), and improved survival (18 months versus 11 months). Similarly, the AZA-001 trial13 showed that, compared to supportive care and low-dose cytarabine (araC), 5-AZA treatment is associated with longer median survival (24.5 months versus 15.0 months; p=0.0001), delayed progression to AML, decreased transfusion requirements, and decreased rate of infections. Decitabine has also demonstrated a modest clinical benefit in clinical trials, with a multicentre Phase II trial (ADOPT)14 showing an ORR of 32%, with 17 patients having complete responses. However, unlike 5-AZA, decitabine has not demonstrated significant survival benefit in a randomised trial.15
Standard AML-based chemotherapy (classically, an anthracycline-araC combination) can also be used in higher-risk MDS, but this therapeutic strategy is associated with significant morbidity, due to prolonged cytopenias and poor long-term survival.16 Therefore, its use is generally limited to younger patients with a favourable karyotype.2,16 Allogeneic stem cell transplantation remains the only curative treatment for higher-risk MDS, and has been associated with prolonged disease-free survival in about 30–50% of patients.17 Although allogeneic stem cell transplantation use has historically been limited to younger patients due to significant toxicities and poor tolerability in older patients,18,19 the emergence of reduced-intensity conditioning allogeneic stem cell transplantation has improved its availability to older patients in recent years.
EMERGING THERAPIES FOR MYELODYSPLASTIC SYNDROMES
Despite the aforementioned advancements, there remain limitations to established therapies. ESA can improve symptoms in low-risk patients with MDS, but do not provide definitive treatment. Lenalidomide has shown efficacy in low-risk MDS with del(5q), but no clear survival benefit has been found. Although HMA have revolutionised the treatment of MDS, only about half of patients treated with HMA achieve objective responses, and most responders eventually lose response within 1–2 years.20 Unfortunately, there remains a lack of therapeutic options for those who fail HMA. Use of conventional chemotherapy in the elderly MDS population is limited by toxicities. Allogeneic stem cell transplantation remains the only curative option, but is limited by donor availability and tolerability. As a result, prognosis for patients with MDS who fail HMA remains poor. For patients with lower-risk MDS who have failed HMA, survival is estimated to be between 14 and 17 months.21 Those with higher-risk MDS who have failed HMA have an even poorer prognosis, with an estimated survival of 4–6 months.22 Fortunately, several novel therapeutics are now being investigated for use in MDS, either as first-line or salvage treatments after HMA failure.
DRUGS TARGETING EPIGENETIC DYSREGULATION
Oral Hypomethylating Agents
Oral azacitidine and decitabine are being explored as alternatives to the more conventional intravenous and subcutaneous administration formulation HMA in ongoing Phase III clinical trials.23 Benefits of an oral formulation include increased patient convenience and the feasibility of an extended drug dosing schedule. Oral azacitidine has already been shown in Phase I and II trials to be bioavailable, clinically active, and well-tolerated, with an ORR, defined as complete response, HI, or TI, of 35% in previously treated patients and 73% in previously untreated patients.24 An extended dosing schedule has also been found to be beneficial, with improved ORR after a 14-day dosing schedule (ORR: 36%) versus a 21-day dosing schedule (ORR: 41%) in those with lower-risk MDS.24
Oral decitabine is the subject of clinical trials as part of a combination agent called ASTX727, which includes a novel cytidine deaminase inhibitor, cedazuridine (E7727). Cedazuridine inhibits cytidine deaminase, thereby inhibiting decitabine degradation in the gut and liver. Initial results from a Phase II study25 of ASTX727 in intermediate and higher-risk MDS were presented at the American Society of Hematology (ASH) Congress 2017, and reported a clinical benefit in 31 out of 50 patients (62%), with 8 (16%) patients in complete remission (CR), 14 (28%) patients with marrow CR, and 9 (18%) individuals with HI.25 A Phase III randomised, open-label, crossover pharmacokinetic study of ASTX727 versus intravenous decitabine is underway to demonstrate comparable exposures of decitabine.
Guadecitabine
Guadecitabine (SGI-110) is a next-generation, subcutaneous, small volume dinucleotide of decitabine and deoxyguanosine currently in clinical trials for higher-risk MDS and AML. Unlike decitabine, guadecitabine is resistant to deamination by cytidine deaminase, allowing prolonged in vivo exposure to the active ingredient decitabine.26-29 A Phase I study has confirmed that a dose of 60 mg/m2 guadecitabine for 5 days is clinically active and well-tolerated in patients with relapsed or refractory MDS or AML that failed HMA.26 Preliminary results of Phase II trials in various MDS cohorts have demonstrated efficacy of guadecitabine as both an initial therapy in higher-risk HMA-naïve MDS patients (ORR: 50–60%)27,29 and as a salvage therapy in higher-risk MDS patients who have failed prior therapy with 5-AZA (ORR: 16%).28 A Phase III randomised open-label study comparing guadecitabine to standard therapy in patients with MDS and chronic myelomonocytic leukaemia after HMA failure is currently underway.30
Histone Deacetylase Inhibitors
Histone deacetylase inhibitors (HDACi) are similar to HMA in that they target epigenetic dysregulation, albeit through a different mechanism. HDACi modulate gene expression by inhibiting the deacetylation of histone lysine tails, relaxing chromatin structure. Secondary mechanisms of action include direct acetylation of nonhistone proteins, alterations to the NFκB signalling pathway, and upregulation of the death receptor pathway.31 Several different HDACi, including sodium phenylbutyrate, valproic acid, romidepsin, vorinostat, mocetinostat, panobinostat, and 4SC-202, have been tested in clinical trials, with many trials still ongoing. Despite robust preclinical data suggesting benefits, Phase I and II clinical trials of HDACi have shown only a modest effect at best.31,32 Notably, none of these agents used alone or in combination with other therapeutic agents have been shown to be superior to HMA monotherapy.33-35
Mutant Isocitrate Dehydrogenase 1 and 2 Inhibitors
Isocitrate dehydrogenase (IDH)1 and IDH2 are enzymes involved in histone demethylation. Recurrent mutations are detected in IDH1/2 genes in about 5% of MDS patients and 20% of AML patients. These are typically gain-of-function mutations that result in increased enzymatic activity, leading to DNA and histone hypermethylation and a blockade of haematopoietic cellular differentiation.36 Several mutant IDH (mIDH) inhibitors are currently being investigated in clinical trials (most of which are still in Phase I or II) for use in patients with relapsed or refractory MDS or AML. The most promising results thus far have been seen with enasidenib (a mIDH2 inhibitor) and ivosidenib (a mIDH1 inhibitor).36 In a recent Phase I and II trial of enasidenib in relapsed and refractory AML, the ORR was 40.3%, with 19.3% of patients achieving CR.37 A significant survival benefit was also demonstrated in those who achieved CR (median OS: 19.7 months in those who achieved CR versus 9.3 months in others).37 Preliminary results of a Phase I and II study, including 17 patients with IDH2-mutated MDS, suggested that enasidenib also has activity in MDS, with a reported OSS of 59%.38 Similarly, ivosidenib has been suggested to have efficacy in patients with advanced haematologic malignancies, including MDS, with 36% ORR in a Phase I study.39
Pyruvate Dehydrogenase and Bromodomain and Extraterminal Domain Inhibitors
Pyruvate dehydrogenase inhibitors (e.g., CPI613)40 and bromodomain and extraterminal domain inhibitors (e.g., CPI-0610) also target epigenetic dysregulation. These inhibitors are currently being studied in Phase I clinical trials with results pending.41
DRUGS TARGETING ABNORMAL SIGNAL TRANSDUCTION
Thrombopoietin Agonists
Thrombopoietin agonists have also been explored for use in lower-risk MDS patients with thrombocytopenia and have shown some benefit. Unfortunately, widespread use has been limited by concern for increased risk of leukaemogenesis. This fear is based on early studies showing a transient increase in blast percentage in 15% of patients treated with high-dose romiplostim, despite a recently published follow-up study reporting no difference in the risk of transformation to AML or mortality with romiplostim versus placebo at 5 years.42 Eltrombopag is an alternative thrombopoietin agonist that has shown efficacy in treating aplastic anaemia and, unlike romiplostim, is not associated with an increase in blast percentage. A recently published Phase II clinical trial (ASPIRE) showed that eltrombopag improved platelet counts and reduced clinically relevant thrombocytopenic events compared to placebo in high-risk MDS and AML patients with thrombocytopenia who are ineligible for other treatment or not receiving disease-modifying treatment.43 Clinical trials to investigate the efficacy of eltrombopag in lower-risk MDS are ongoing; however, preliminary results show efficacy in improving thrombocytopenia and reducing bleeding events in this population as well.44
Transforming Growth Factor-β Signalling Modulators
TGF-β signalling modulators have been developed for treatment of MDS with the aim of inhibiting TGF-β-meditated myelosuppression,45 thereby promoting maturation of haematopoietic progenitors in bone marrow. Luspatercept (ACE-536) is a selective activin receptor ligand that traps GDF11, blocking TGF-β signalling and promoting late-stage erythropoietic maturation. It has shown promising activity in preliminary results of an ongoing Phase II multicentre study in patients with lower risk MDS with anaemia (PACE-MDS): 53% of patients showed erythroid response and 38% of patients achieved TI.46 In this study, particularly high response rates were observed among patients with refractory anaemia with ring sideroblasts, SF3B1 mutation, and/or low transfusion burden, leading to the development of a randomised trial of luspatercept for patients with refractory anaemia with ring sideroblasts.47 Sotatercept (ACE-011), which functions through a very similar mechanism, has also shown promising evidence of clinical activity in ESA-refractory lower-risk MDS patients in an ongoing Phase II study, with 49% of patients showing improvement in anaemia.48 Galunisertib, an agent that inhibits the kinase activity of TGF-β receptor Type 1, also known as ALK5, is also currently in Phase II and III clinical trials, with preliminary results showing HI in 21% of the 38 lower-risk MDS patients studied.49
Toll-Like Receptor Inhibitors
TLR signalling is abnormally activated in MDS, especially after HMA therapy, leading to activation of the NF-κB pathway and inhibition of haematopoiesis.50 OPN-305 is a fully humanised IgG4κ monoclonal antibody against TLR2 that is currently being investigated in Phase I and II clinical trials for the treatment of low or INT-1 risk MDS after HMA failure.51 Preliminary results in 21 patients have shown haematologic improvement in 53% of patients, with 20% achieving TI.52 Further studies are necessary to confirm this response and evaluate effect on survival.
Rigosertib
Rigosertib is a multikinase inhibitor that induces mitotic arrest and apoptosis in neoplastic cells while sparing normal cells. It has been explored for use in patients with refractory MDS with excess blasts or treatment-related MDS who have failed HMA.53 In a Phase III study (ONTIME)54 comparing rigosertib to best supportive care with or without low-dose araC in this population, rigosertib was not shown to improve OS. However, in a post-hoc analysis, patients with very high-risk IPSS-R scores and monosomy 7 or trisomy 8 were found to have significant improvement in survival with rigosertib compared to supportive care (median OS: 7.6 months versus 3.2 months, p=0.015).54 A second Phase III study in this smaller cohort of very high-risk patients is now underway to further evaluate this effect.55
FMS-like Tyrosine Receptor Kinase Inhibitors
Midostaurin is a FMS-like tyrosine receptor kinase (FLT) 3 inhibitor that was recently approved for use in combination with 7+3 chemotherapy for patients with newly diagnosed FLT3 mutation-positive AML, based on clinical trial data showing improved OS and event-free survival in patients treated with midostaurin.56 Though not formally approved for use in MDS as of yet, FLT3 inhibitors (i.e., midostaurin, sorafenib) have also demonstrated efficacy in Phase I and II clinical trials when used in combination with HMA (i.e., 5-AZA, decitabine) in patients with FLT3-mutated MDS.57,58 Notably, although FLT3 mutations are seen in <1% of patients with newly diagnosed MDS, they have been observed in up to 5% of patients at the time of MDS transformation to AML and are associated with much poorer outcomes.59 The hope is that mutation-targeted therapy will improve these outcomes.
OTHER NOVEL THERAPEUTICS
Immune Checkpoint Inhibitors
Several monoclonal antibodies against immune checkpoint regulators, both alone and in combination with HMA, are being tested in clinical trials. These include nivolumab (a programmed cell death protein [PD-1] inhibitor), ipilimumab (a CTLA-4 inhibitor), pembrolizumab (a PD-1 inhibitor), and durvalumab (blocks interaction of PD ligand-1 with PD-1 and CD80 molecules). Early results of these studies have been mixed. Preliminary results from a Phase Ib study of pembrolizumab in 27 patients with MDS after HMA failure (KEYNOTE-013) show only modest response to therapy, with no CR, 1 partial remission, 3 marrow CR, and 3 HI.60 However, 2-year survival was reportedly 57% in those treated with pembrolizumab, which is considerably superior to that typically expected in MDS patients after HMA failure.60 A Phase II study evaluating nivolumab and ipilimumab as single agents or in combination with AZA for patients with MDS (as initial or salvage therapy) is also ongoing, with preliminary results in 39 patients showing an ORR of 69% in the AZA plus nivolumab cohort, 22% in the ipilimumab monotherapy cohort, and no response in the nivolumab monotherapy cohort.61 Further follow-up is needed to clarify efficacy and safety.
Proteasome Inhibitors
Proteasome inhibitors inhibit cell death by preventing degradation of proapoptotic proteins. The proteasome inhibitor, bortezomib, has recently been explored as a therapeutic option in MDS. In a limited study of 15 patients with low or INT-1 MDS after HMA failure, bortezomib showed only modest effect, with HI observed in 20% of patients.62 However, an unexpected significant reduction of ring sideroblasts was seen in 70% of patients treated with bortezomib in this study, suggesting potential benefit specifically in MDS patients with ring sideroblasts.62 Further studies are necessary to better evaluate this effect.
B Cell Lymphoma-2 Inhibitors
Antiapoptotic resistance due to B cell lymphoma (BCL)-2 overexpression has been reported in higher-risk MDS and may contribute to disease pathogenesis. As a result, BCL-2 inhibitors have been explored as a therapeutic option in higher-risk MDS after HMA failure and in relapsed and refractory AML. Venetoclax is the best studied BCL-2 inhibitor thus far, and has shown modest efficacy (ORR: 19%) as monotherapy in patients with high-risk relapsed or refractory AML.63 Venetoclax has also shown significant efficacy in combination with araC for patients with treatment-naïve AML (including those with previously treated MDS) in a Phase I trial of 20 patients, with 14 out of 20 patients (70%) achieving CR or CR with incomplete marrow recovery.64 The presence of mutations in chromatin-RNA splicing genes (ASXL1, EZH2) or NPM1 correlated with a higher likelihood of response in a separate analysis.65 A Phase I clinical trial evaluating venetoclax alone and in combination with azacitidine in higher-risk MDS after HMA failure is ongoing.66
RNA Splicing Modulators
Genes encoding various spliceosome components (SF3B1, SRSF2, U2AF1, and ZRSR2) are frequently mutated in patients with MDS.67,68 While the precise mechanism by which abnormal RNA splicing leads to the development of MDS is unknown, the association is clear. Based on this knowledge, H3B-8800, a potent and orally bioavailable SF3b complex modulator, was developed and is now being studied in a Phase I clinical trial for use in patients with MDS and AML with splicing mutations, with results pending.69,70
Cytotoxic Agents
Although many patients with MDS cannot tolerate standard AML-based chemotherapy, less intensive cytotoxic agents can be considered. Cytotoxic agents that have been explored for use in MDS patients include low-dose clofaribine and CPX-351. Low-dose clofarabine is currently under investigation for both higher-risk MDS and AML, with promising results. A Phase II study evaluating the safety and efficacy of low-dose clofaribine in combination with araC in patients with MDS who have failed HMA has reported an ORR of 44%, median OS of 10 months overall, and median OS of 22 months in responding patients (versus 4 months in non-responding patients).71 CPX-351, a liposomal formulation of araC and daunorubicin in a 5:1 molar ratio, was recently U.S. Food and Drug Administration (FDA) approved as front-line therapy for patients with therapy-related AML and AML with MDS-related changes based on a Phase III randomised trial reporting a survival advantage compared to standard 7+3 chemotherapy (OS: 9.6 months versus 5.9 months).72 However a subsequent Phase II randomised trial testing attenuated doses of CPX-351 in a small cohort of less fit adults with untreated AML or MDS with 10% or more blasts in peripheral blood or bone marrow did not show much efficacy.73
CONCLUSION
Although HMA have changed the landscape of MDS therapy in the past decade, they have limitations and are not curative. There remains a significant proportion of patients who simply do not respond to HMA, or who develop resistance to HMA after initial response. Unfortunately, established therapeutic options for patients with MDS who have failed HMA therapy are limited, and these patients have very poor prognosis. Thus, multiple novel agents are being developed in clinical trials with the aim of improving symptoms and OS in patients with MDS.







