Abstract
Multiple myeloma is a condition that affects predominantly the older population. There are now various approved chemotherapy regimens as a result of advances in treatment. Choosing the optimal regimen for older patients with myeloma remains a challenge because of frailty and a lack of head-to-head comparisons between backbone regimens. The purpose of this literature review is to summarise the recent literature on frailty assessment, disease biology, and treatment efficacy in the frontline and relapsed settings to aid the decision-making process.
INTRODUCTION
Multiple myeloma is a haematological malignancy predominantly affecting older people and has a median age of onset of 70 years.1 Traditionally, the term ‘elderly’ in myeloma applied to those aged over 65–70 years, as these patients were considered as transplant-ineligible. However, this is a heterogeneous group of patients with varying degrees of fitness, physiological reserve to tolerate treatment, and life expectancy. In recent years, there have been renewed interests in this group of patients, with specifically designed prospective trials to determine treatment outcomes and tolerability, and research on the impact of frailty amongst these patients. This review aims to summarise key findings from the literature on older patients with myeloma, including frailty assessment, disease biology, and chemotherapy treatment in the frontline and relapsed settings.
METHODS
A literature search using the keywords “elderly”, “frailty”, “myeloma”, and “transplant-ineligible” was conducted in Google Scholar, PubMed, and Medline. Articles written in English were included and a preferential focus was placed on Phase III clinical trials and systematic reviews that were published within the last 10 years. Preliminary analyses, Phase I/II trials, post hoc analyses, and commentaries were included if they contained relevant merits.
FRAILTY ASSESSMENT
Frailty is a cumulative state of physiological decline associated with ageing. It has been reported that frail individuals have a reduced physiological reserve to external insult such as chemotherapy.1,2 One of the earlier definitions of frailty was described by Fried et al.3 as having three of the following five components: unintentional weight loss, poor grip strength, self-reported exhaustion, slow mobility, and a low level of physical activity. Since then, several surrogate scoring tools have been developed for predicting frailty amongst patients with myeloma (Table 1).
The International Myeloma Working Group (IMWG) frailty assessment tool was developed based on a cohort of 869 transplant-ineligible (TI) patients with myeloma (Table 1).4 The frail score was composed of age categories, Katz Activity of Daily Living (ADL), Instrumental Activity of Daily Living (IADL), and Charlson Comorbidity Index (CCI). Three groups were identified: fit (score of 0), intermediate fit (score of 1), and frail (score of ≥2). This tool was predictive of 3-year overall survival (OS) (84% in fit; 76% in intermediate fit; and 57% in frail patients), serious treatment-related events, and incidence of treatment discontinuation.4 The Revised Myeloma Comorbidity Index (R-MCI) is an updated version of the Initial Myeloma Comorbidity Index, with additional information on adverse cytogenetics and frailty assessment (Table 1).5 The score was internally validated to predict median OS (10.1 years in the fit group; 4.4 years in the intermediate fit group; and 1.2 years in the frail group).5
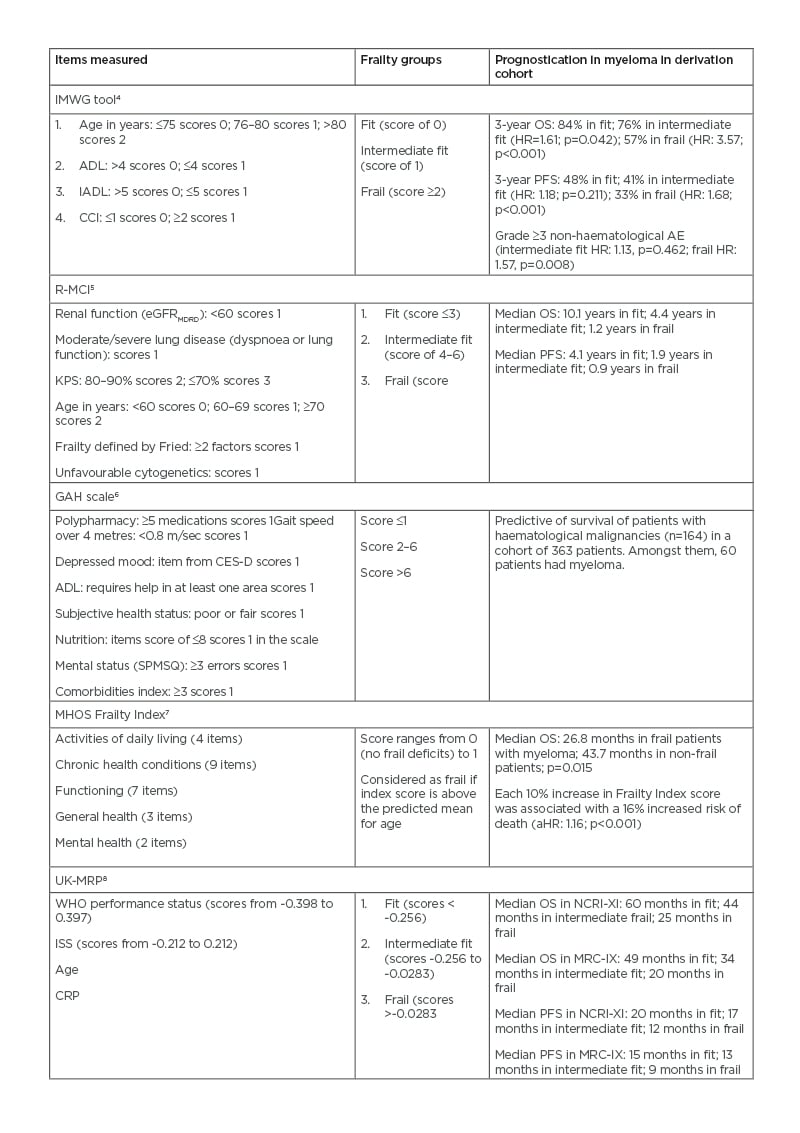
Table 1: Frailty assessment tools in multiple myeloma.
ADL: Katz activity of daily living; AE: adverse event; aHR: adjusted HR; CCI: Charlson Comorbidity Index; CES-D: Center For Epidemiologic Studies Depression Scale; CRP: C-reactive protein; eGFRMDRD: estimated glomerular filtration rate calculated using the modification of diet in renal disease study equation; GAH: Geriatric Assessment in Hematology; HR: hazard ratio; ISS: international staging system; MHOS: Medicare Health Outcomes Survey; MRC-IX: Medical Research Council Myeloma IX trial; NCRI-XI: National Cancer Research Institute Myeloma XI study; IADL: instrumental activity of daily living; IMWG: International Myeloma Working Group; KPS: Karnofsky performance status; OS: overall survival; PFS: progression-free survival; R-MCI: Revised Myeloma Comorbidity Index; SPMSQ: Short Portable Mental Status Questionnaire; UK-MRP: UK Myeloma Research Alliance Risk Profile; WHO: World
Health Organization.
It remained predictive of survival outcomes in the younger subgroup (age <65 years).5 This emphasised that frailty was not limited to older patients.
These tools are not without shortfalls. The ADL and IADL assessments of the IMWG tool can take on average 15–20 minutes to complete, which affects its adoption in day-to-day clinical practice.9 Meanwhile, the score appeared to have limited ability to predict OS when applied to a separate cohort of patients aged ≥75 years.10 There was also inconsistency across different frailty tools, with the IMWG tool classifying more patients into the frail category than R-MCI and CCI.11
Simplified tools have been proposed to address the time constraint issue in frailty assessment at a day-to-day clinical practice. The first group, including the Geriatric Assessment in Haematology (GAH) Scale and the Medicare Health Outcomes Survey (MHOS) Frailty Index, were based on self-reported elements (Table 1). The former was a 30-item list shown to predict mortality in haematology patients.6 The latter derived 25 questionnaire items on ADL, chronic health conditions, functional status, subjective general health, and mental health. Every 10% increase in the frailty index predicted a 16% increased risk of death in patients with newly diagnosed myeloma aged >65 years.7 Although these surveys can be completed before clinic attendance, they rely heavily on patients’ subjective assessment, which can be a source of bias.
Another approach is to use performance-based assessment such as gait speed, which can be easily obtained using the 4 m gait speed test developed by the National Institutes of Health (NIH).12 In a recent analysis of patients aged ≥75 years with a haematological malignancy, reduced gait speed was associated with increased mortality, hospitalisation, and emergency visits.13 In a study of prognostication in patients with myeloma, performance status (PS) consistently contributed to survival outcomes regardless of their age groups.14 A simplified frailty score based on a patient’s age, CCI, and Eastern Cooperative Oncology Group (ECOG) PS was retrospectively analysed from the FIRST trial cohort and showed frail patients experienced worse PFS, OS, and more treatment-related adverse events.15 The performance of this score was validated in an independent dataset from the HOVON87/NMSG18 study with 636 TI patients.16 Similarly, in a post hoc analysis of the ASPIRE and ENDEAVOR trials, frailty score based on age, CCI, and PS appeared to show worse PFS and OS in frail inviduals.17 Some studies explored the use of biomarkers in frailty assessment. For example, the UK Myeloma Research Alliance Risk Profile (UK-MRP) was based on the non-intensive arms of NCRI-Myeloma-XI and MRI-IX trials and found that World Health Organization (WHO) PS, International Staging System (ISS), age, and C-reactive protein were associated with progression-free survival (PFS), early mortality, and the percentage of treatment dose delivered (Table 1).8 Although these models appeared to show prognostic significance, some weighed more heavily towards disease characteristics. PS-based assessments are convenient for use in a day-to-day clinical setting; however, they may not encompass the full spectrum of the frailty phenotype and can be subjective.18
Despite a lack of consensus on an ideal frailty assessment tool in a day-to-day clinical practice, the above data indicated that some form of frailty assessment would provide prognostic information on older patients with myeloma. Questions remain on tailoring treatment based on frailty; hopefully, data from the FRAIL-M study and the UK MRC-XIV study19 will provide further insight.
FRONTLINE THERAPY
Novel agents such as bortezomib and lenalidomide had been established as the standard of care. The addition of bortezomib to melphalan and prednisolone (VMP) for TI patients in the VISTA study showed survival benefit even in those aged ≥75 years (Table 2).20 A reduced once-weekly bortezomib dosing showed a reduction of neuropathic and gastrointestinal adverse effects without affecting efficacy.31 Meanwhile, continuous lenalidomide and dexamethasone (Rd) became an established regimen after demonstrating superior PFS and OS over the previous standard of care of a melphalan, prednisolone, and thalidomide regimen in the FIRST trial (Table 2).22,32 Addition of an alkylating agent to the Rd regimen did not show superiority in the EMN01 study unless the patient was classified as fit according to the IMWG tool.23,33
Regimens combining immunomodulatory drugs (IMiD) and bortezomib were investigated for their synergistic effect. Bortezomib and thalidomide combination (VTP) demonstrated a higher incidence of Grade ≥3 cardiac complications (8% versus 0% in the VMP group), offsetting the potential benefit.34 In the UPFRONT study, bortezomib, thalidomide, and dexamethasone (VTD) did not show superior OS to the VMP regimen but more neuropathy, whilst the doublet combination of bortezomib and dexamethasone had comparable efficacy (Table 2).24 Using lenalidomide with bortezomib appeared to be more favourable, with better tolerance than thalidomide. The combination of lenalidomide, bortezomib, and dexamethasone demonstrated superior survival outcome and duration of response than Rd alone in the SWOG-S0777 trial (Table 2).25 However, the study population was generally younger (median age of 63 years) than what was considered as ‘elderly’, and 10% proceeded for autologous transplant. Subgroup analysis from the SWOG-S0777 trial demonstrated significant overall survival benefit but not PFS benefit in those aged >75 years, whilst neurological toxicity was significantly more prevalent with bortezomib (33% versus 11% in Rd).25
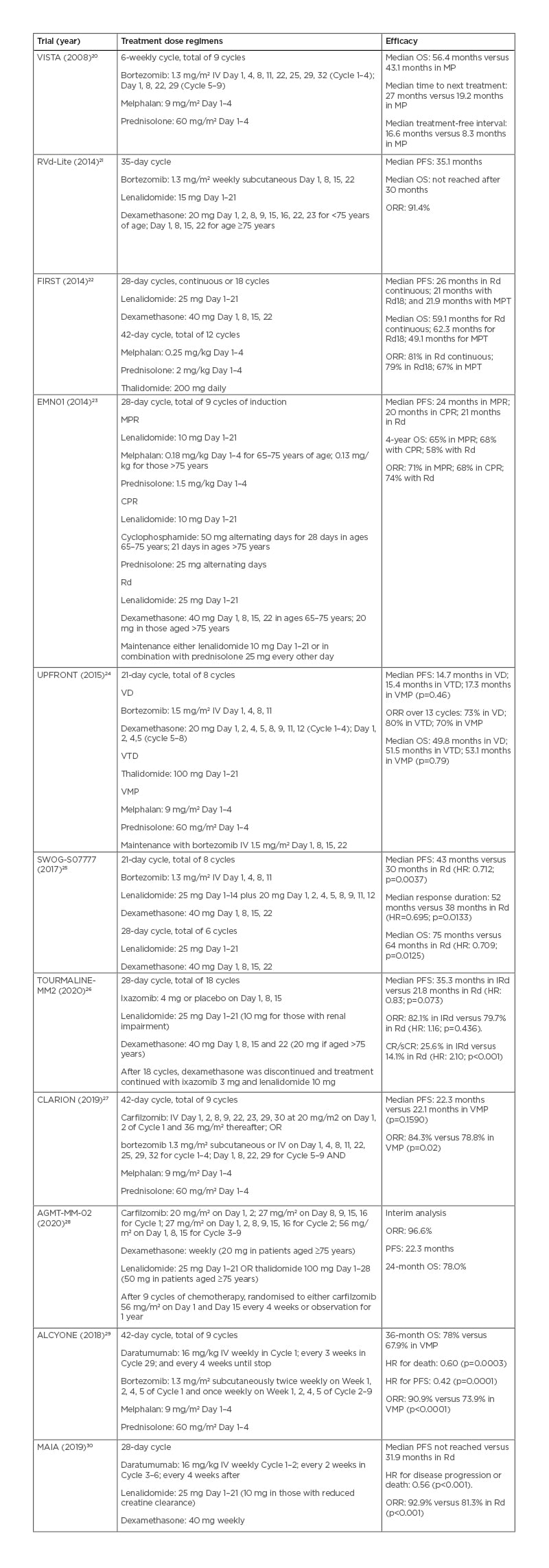
Table 2: Upfront chemotherapy treatment, dosage regimens, and efficacy in older patients with myeloma.
CPR: cyclophosphamide, prednisolone, and thalidomide; CR/sCR: complete response/stringent complete response; HR: hazard ratio; IRd: ixazomib, lenalidomide, and dexamethasone; IV: intravenous; MP: melphalan and prednisolone; MPR: melphalan, prednisolone, and lenalidomide; MPT: melphalan, prednisolone, and thalidomide; ORR: objective response rate; OS: overall survival; PFS: progression-free survival; Rd: lenalidomide and dexamethasone; Rd18: lenalidomide and dexamethasone for 18 cycles; VD: bortezomib and dexamethasone; VMP: bortezomib, melphalan, and prednisolone; VTD: bortezomib, thalidomide, and dexamethasone.
In another study, O’Donnell et al.21 demonstrated this regimen can be dose-reduced in older patients (bortezomib: 1.3 mg/m2 subcutaneous weekly; lenalidomide: 15 mg on Day 1–21; and dexamethasone: 20 mg) and achieved comparable PFS of 35 months (Table 2).21 Only one patient from a cohort of 50 experienced Grade 3 peripheral neuropathy.21 These data support a bortezomib and lenalidomide combination as an efficacious upfront treatment option for fit, older patients with myeloma, which can be dose-adjusted to increase tolerability.
Carfilzomib was combined with melphalan and prednisolone in the CLARION study for the TI group against VMP. There were no significant differences between the two regimens in the median PFS, time to progression, response rate, median duration of response, and measurable residual disease (MRD) (Table 2). More patients in the regimen containing carfilzomib discontinued treatment because of adverse effects (16.7% versus 14.7%), while the VMP regimen had a high incidence of neuropathy (35.1% versus 2.5%; p<0.0001). The authors suggested once-weekly carfilzomib rather than twice weekly might increase its tolerability.27 A recent interim analysis from the AGMT-MM-02 study showed that older TI patients with myeloma who received twice weekly carfilzomib for two cycles followed by weekly carfilzomib at 56 mg/m2 from Cycle 3 to 9 in combination with IMiDs and dexamethasone experienced high objective response rate (ORR; 96.6%), whilst, 47.1% of participants had achieved deep response as measured by negative MRD (Table 2).28
Daratumumab, an anti-CD38 antibody, is emerging as a frontline treatment candidate. The addition of daratumumab to VMP showed higher response rates, higher incidence of negative MRD, prolonged PFS, and better OS in the ALCYONE study (Table 2).29,35 Meanwhile, the MAIA study investigated the addition of daratumumab to Rd in TI patients. It showed a superior event-free survival, ORR, and negative MRD status (Table 2).30 Both trials demonstrated higher infection rates in the daratumumab groups, especially respiratory infections.29,30,35 In a recent meta-analysis of upfront regimens for older TI patients with myeloma, daratumumab combinations appeared to be the most efficacious in
prolonging PFS.36
The aforementioned frailty assessment may help determine the optimal frontline chemotherapy regimen for older patients with myeloma. Fit patients appeared to benefit from lenalidomide triplet as opposed to Rd alone based on data from a post hoc analysis of the EMN01 trial.33
Although there was no head-to-head comparative trials, a pooled analysis by Larocca et al.37 compared bortezomib-based regimens against Rd (with maintenance lenalidomide). It showed no difference in survival outcomes for patients with standard-risk cytogenetics but improved outcomes for those with high-risk cytogenetics receiving VMP. Interestingly, those aged >75 years with standard cytogenetics appeared to gain benefit from the Rd-based regimen as opposed to the younger counterparts who benefited more from VMP.37
Consideration of treatment toxicity profile can influence the choice of regimens. Continuous Rd in the FIRST trial showed a lower rate of neutropenia (30% versus 45% in melphalan, prednisolone, and thalidomide); however, a longer period of chemotherapy exposure might contribute to a higher rate of Grade 3 or 4 infections (32% versus 17% in melphalan, prednisolone, and thalidomide).32 Cardiac toxicity from the VTP regimen would limit its tolerability in older patients as they are more likely to have pre-existing cardiac comorbidities.34 Peripheral neuropathy and gastrointestinal toxicity from bortezomib can be less tolerated in older and frail patients. These issues continue to encourage studies of new chemotherapy regimens for older patients with myeloma.
Ixazomib is an oral proteasome inhibitor with the advantages of less hospital attendance for infusion and the potential for use as a maintenance treatment. The results from the Phase III TOURMALINE-MM02 study in TI patients with myeloma showed a median PFS of 35.3 months in the ixazomib, lenalidomide, and dexamethasone arm compared with 21.8 months in the Rd arm (hazard ratio: 0.83; p=0.073); however, the results failed to reach statistical significance (Table 2).26 In a Phase II HOVON 143 study, a combination of ixazomib, daratumumab, and dexamethasone were studied in unfit and frail patients with myeloma who were classified according to the IMWG criteria. Interim efficacy analysis showed ORR of 87% and 78% in the unfit and frail groups, respectively.38 Elotuzumab, a monoclonal antibody against SLAMF7, in addition to Rd was recently studied in the ELOQUENT-1 trial. It did not show a significant difference in PFS against Rd.39 The use of belantamab mafodotin, an antibody–drug conjugate, was studied in the relapsed and refractory settings.40 The DREAMM-9 study is designed to specifically study its efficacy and safety in combination with bortezomib, lenalidomide, and dexamethasone in TI participants.41
QUALITY OF LIFE
Despite the increasing use of novel agents and monoclonal antibodies in older patients with myeloma, it remains an incurable disease with poor survival outcomes. Measurement of the quality of life must be considered in this group. In the FIRST trial, continuous Rd improved health-related quality of life during the first 18 months, which might be maintained beyond this timeframe if treatment continued.42 In the HOVON-87/NMSG18 study, health-related quality of life was assessed using the EORTC QLQ-C30 and MY20 questionnaires at baseline, induction, and maintenance therapies.43 Improvement in the quality of life was evident in the lenalidomide-based treatment group during the maintenance phase as opposed to a higher incidence of neuropathy with thalidomide maintenance, which offset its potential benefit in reducing disease progression. In the CLARION study, quality of life measures appeared to favour carfilzomib, especially in the domains of physical function, fatigue, pain, and treatment side effects.27 In the RVD-lite cohort, patients receiving a reduced-intensity triplet regimen experienced significant improvements in physical function, future perspective, and disease symptoms.21 Complications from skeletal and extramedullary diseases would impact on the quality of life in myeloma patients; therefore, radiation treatment, anti-resorptive therapies, and early access to palliative care would likely influencepatient outcomes.
CYTOGENETICS IN TRANSPLANT-INELIGIBLE PATIENTS
It is recognised that older patients with myeloma have a different tumour genetic profile. The proportion of patients with t(4;14) and del(17p) appeared to lessen in the older age group, while the opposite was observed for those with gain(1q). Molecular study data showed that mutational signatures associated with hyperdiploidy were common with ageing.44 The impact of t(4;14) and gain(1q) were less in the very old group (>80 years), while del(17p) persistently predicted adverse outcomes across different age groups.14 The overall impact became smaller with advancing age.14 Poor PS and frailty predominated over adverse cytogenetic markers in prognosticating those >80 years.14 This illustrates that genetic markers are not uniform in their prognostic impact with advancing age.
Questions remain as to how best to tailor frontline treatment in TI patients based on the cytogenetic results in the absence of well-designed, risk-adapted studies. A recent post hoc analysis by Larocca et al.45 compared outcomes between bortezomib- and lenalidomide-based treatment arms from the GIMEMA-MM-03-05 and EMN01 trials based on the cytogenetic results. It showed that bortezomib could overcome cytogenetic adversity of t(4;14) and t(14;16) in TI patients to gain PFS benefit.45 This was not apparent in those with del(17p).
AUTOLOGOUS TRANSPLANT
Data on the role of autologous stem cell transplant in older patients with myeloma are not as robust because of the use of age threshold and PS as surrogate markers for transplant eligibility. The IFM-99-06 was a prospective study on autologous transplant in older patients.46 There was no significant survival benefit when comparing reduced-intensity conditioning autologous transplant with a melphalan plus prednisolone regimen. On the other hand, older patients with myeloma showed comparable benefits from autologous transplant to the younger patients.47
Non-relapse mortality (NRM) was thought to be higher in patients over the age of 70 years with a conventional melphalan dose of 200 mg/m2. Trials using a reduced-intensity regimen showed improved outcomes in older patients with myeloma without reducing their efficacy.48,49 This has been challenged by an analysis of the CIBMTR database, which included 15,999 patients with myeloma during 2013–2017.50 Age ≥70 years was not demonstrated to have significantly worse NRM (hazard ratio: 1.3; 99% confidence interval: 1.0–1.7). Furthermore, older patients who received reduced dose conditioning had worse outcomes than those receiving a full dose in aspects of Day-100 NRM, 2-year PFS, and 2-year OS.50
A more comprehensive approach in selecting transplant candidates is needed than using biological age alone. A Haematopoietic Cell Transplantation Comorbidity Index (HCT-CI) ≥3 was not associated with a worse survival outcome.51 Using the IMWG classification tool, frailty was associated with cumulative gastrointestinal toxicity and infections.52
Overall, efficacy data appeared to support autologous transplant in fit and older patients with myeloma. With the emergence of efficacious and novel chemotherapy regimens, it is unclear whether this efficacy remains relevant in older patients with myeloma.
RELAPSED OR REFRACTORY TREATMENT
Outcomes in older patients with either relapsed or refractory myeloma remain poor, and there were few studies dedicated to this group. Data guiding current management in the relapsed or refractory settings were mostly based on subgroup analyses of clinical trials.
Newer IMiD such as pomalidomide showed efficacy in the relapsed or refractory settings.53 In the MMWP-164 study, Lee et al.54 investigated the efficacy of pomalidomide with cyclophosphamide and dexamethasone in older patients with myeloma. The outcome appeared decimal as only 10% of patients remained on treatment at the last follow-up, mostly because of disease progression.54 The median PFS was 6.9 months and an OS of 18.49 months was recorded.54 Guarded outcomes were also observed in younger patients receiving pomalidomide after they experienced relapsed or refractory disease.55
The ENDEAVOR trial showed that carfilzomib and dexamethasone were efficacious in the relapsed or refractory settings.56 In the subgroup analysis of the 17% of patients aged ≥75 years, median PFS was 18.7 months in the carfilzomib group as opposed to 8.9 months in the bortezomib group.57 Median PFS in the younger subgroup receiving carfilzomib was 15.6 months, suggesting older patients might benefit more from carfilzomib.57 Carfilzomib was combined with lenalidomide and dexamethasone in the ASPIRE trial; however, no statistically significant difference in PFS was noted in the ≥65 age group when compared with Rd alone.58 This might be because of a higher discontinuation rate from cardiac toxicity in the carfilzomib group.
Despite recent studies focused on the use of ixazomib in the upfront setting, it was commonly used in the relapsed or refractory settings. In the TOURMALINE-MM1 study, ixazomib was added to the Rd backbone, demonstrating a superior medial PFS of 20.6 months as opposed to 14.7 months.59 However, the statistical difference has not been demonstrated in the older age group.
The potential role of isatuximab, an anti-CD38 monoclonal antibody, was supported by the ICARiA-MM study when it was used in conjunction with pomalidomide and dexamethasone.60 A significant proportion (65%) of the participants who received the intervention were aged ≥65 years. Overall, this resulted in a median PFS of 11.53 months compared with 6.47 months in
the control.60
There are reasonable data on daratumumab in the relapsed or refractory settings. The CASTOR study investigated the combination of daratumumab and bortezomib, showing potential benefits for those aged ≥65 years.61 When used with lenalidomide and dexamethasone, it was efficacious in those aged 65–74 years but not those aged ≥75 years.62 The CANDOR study investigated the combination of daratumumab and carfilzomib and found no PFS benefit in those aged >65 years, potentially because of its adverse effects.63 The emerging data on daratumumab in the upfront setting may phase out its use in the relapsed or refractory settings.
Elotuzumab used in junction with lenalidomide and dexamethasone was evaluated in the ELOQUENT-2 trial.64 Survival benefit had been shown in those aged ≥75 years. When used in conjunction with pomalidomide and dexamethasone, median PFS and OS were prolonged; however, this had not been observed in the older age group.65
Although chimeric antigen receptor T cells have emerged as a novel immune therapy for heavily pre-treated patients with myeloma, this has not been studied in the older patients. It is worth noting that pre-treatment lymphodepleting agents, such as cyclophosphamide or fludarabine, and dose-related effects, including cytokine release syndrome and neurotoxicity, may limit its use in those with pre-existing age-related organ dysfunction, comorbidities, or frailty.
Despite novel agents, treatments in the relapsed or refractory settings for older patients with myeloma were mostly based on subgroup analyses in the large clinical trials. These were not necessarily representative of the real-world clinical scenarios of a significant proportion of older patients, who were likely to be frailer and less likely to tolerate salvage treatment.
CONCLUSION
Myeloma is a chronic disease of older people, with poor survival outcomes and quality of life. Older and frail patients are less able to tolerate treatment and more likely to experience disease progression. Although recent data on the novel chemotherapy agents showed promising results, it remains vital to consider frailty status, treatment toxicity, and quality of life when deciding on a management plan. In the authors’ review, several validated frailty assessment tools can predict survival outcomes. However, their use in a clinical setting requires an extensive amount of expertise and time. The authors recommend screening older patients with simplified or performance-based tools at the first instance and, if resources permit, conducting a detailed frailty assessment. Fit, older patients with myeloma should be offered highly efficacious treatment regimens and considered for autologous stem cell transplant. Frail, older patients with myeloma should be offered a balanced approach. Moreover, continuous oral therapy such as lenalidomide may be favoured over parenteral proteasome inhibitors. Dose reduction should be considered if toxicity limits treatment tolerability as there is emerging evidence of highly efficacious dose-reduced regimens. Early palliative care involvement and supportive measures form important aspects in managing older patients with myeloma but they are outside the scope of this review.







