Medical Writer: Jennifer Taylor
Acknowledgements: Medical writing assistance was provided by Jennifer Taylor, London, UK.
Support: The writing and publication of this article was funded by Incyte Biosciences.
Received: 26.06.20
Accepted: 23.07.20
Citation: EMJ Hematol. 2020;8[Suppl 4]:2-4.
Abstract:
The fourth iteration of the European LeukemiaNet (ELN) recommendations for treating chronic myeloid leukaemia (CML) were published in early 2020.1 This update of the 2013 version2 is a result of new developments and research, which have led to dramatic changes in the therapeutic landscape of CML.
This article provides an overview of the CML recommendations that are relevant for the third-generation tyrosine kinase inhibitor (TKI) ponatinib (Iclusig®).3 The authors state that in second-generation TKI-resistant patients without specific mutations, ponatinib is preferred over another second-generation TKI, unless cardiovascular risk factors preclude its use.4,5
SUMMARY OF 2020 RECOMMENDATIONS ON PONATINIB
Ponatinib is indicated for the treatment of adults with chronic myeloid leukaemia (CML) who are resistant to dasatinib or nilotinib, are intolerant to dasatinib or nilotinib and for whom subsequent treatment with imatinib is not clinically appropriate, or have the T315I mutation.3 Ponatinib is not approved for paediatric use.
The recommendations state that in cases of resistance to the initial second-generation tyrosine kinase inhibitor (TKI), given either as first- or second-line therapy, it is unlikely that the patient will achieve a durable response to an alternative second-generation TKI. The 2020 updated European LeukemiaNet (ELN) guidance therefore recommends that patients who are resistant to a second-generation TKI and do not have specific mutations should be treated with ponatinib instead of another second-generation TKI, unless cardiovascular risk factors preclude its use. An experimental agent should also be considered, and the patient should be assessed for allogeneic stem cell transplantation. The 2020 recommendations, similar to the previous 2013 guidance, recommend ponatinib for patients with the T315I BCR-ABL1 resistance mutation, as it is currently the only TKI with activity against this mutation.
The approved starting dose of ponatinib is 45 mg once daily, regardless of the CML phase or line of treatment.3 This is reflected in the ELN guidelines, which do not suggest a different dosage of ponatinib accordingly to disease phase or line of treatment. However, in the new edition of the recommendations, the panel advises starting at 30 mg or 15 mg daily for patients with a low degree of resistance or multiple intolerances, especially those with an increased cardiovascular risk profile. According to the updated panel recommendations, patients with the T315I mutation, compound mutations, or progression to an advanced phase should start with ponatinib 45 mg once daily. Data from preliminary studies indicate that the daily dose can be reduced to 15 mg daily if complete cytogenetic remission or major molecular response is achieved. Disease and toxicity should be closely monitored.
Patients who do not respond to ponatinib after 3 months are likely at high risk of progression and the panel advises that they should undergo assessment for allogeneic stem cell transplantation.
CARDIOVASCULAR RISK
Thought should be given to cardiovascular risk when considering use of ponatinib because prior or current arterial disease may be a contraindication to ponatinib treatment in the second- or third-line. Of all the TKI, ponatinib carries the highest risk of developing an arterial occlusion event.
Numerous steps can be taken to facilitate the use of ponatinib in patients with increased cardiovascular risk. The 2020 ELN recommendations advise to start ponatinib at a reduced dose (30 mg or 15 mg daily); control hypertension, hyperlipidaemia, and diabetes; and cease smoking. The benefit of prophylactic aspirin or anticoagulation is unclear.
IDENTIFYING FAILURE EARLY
Molecular response should be assessed using the International Scale (IS) as the ratio of BCR-ABL1 transcripts to ABL1 transcripts and stated as BCR-ABL1 %. Monitoring milestones at 3, 6, and 12 months are used to decide whether the current treatment should be continued (optimal response); carefully considered for continuation or change, depending on patients’ characteristics, comorbidities, and tolerance (warning); or changed (failure/resistance) (Table 1). Treatment has failed when BCR-ABL1 at 3 months is greater than 10%.
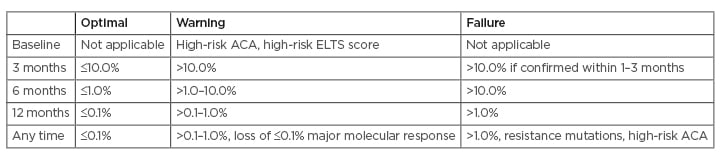
Table 1: Milestones for treating chronic myeloid leukaemia expressed as BCR-ABL1 on the International Scale (IS).
Definitions of response applicable to first- and second-line treatment.
A change of treatment may be considered if a major molecular response is not reached by 36–48 months.
ACA: additional chromosome abnormalities in Philadelphia chromosome-positive cells; ELTS: European Treatment and Outcome Study (EUTOS) long-term survival score.
Adapted from Hochhaus et al.1
Where there is failure/resistance, a change of TKI is mandatory and BCR-ABL1 kinase domain mutations should be assessed using next-generation sequencing. The TKI selection should then be guided by the profile of BCR-ABL1 kinase domain mutations, especially if the T315I mutation is detected, as only ponatinib is an effective treatment (Table 2).
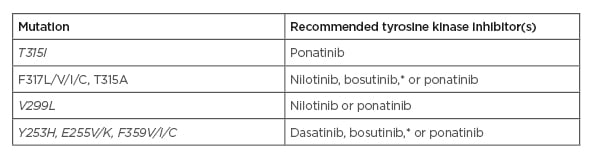
Table 2: Recommended tyrosine kinase inhibitors in case of BCR-ABL1 resistance mutations.
*There are limited data available regarding mutations associated with clinical resistance to bosutinib in vivo. Some in vitro data suggest that the E255K and, to a lesser extent, the E255V mutation might be poorly sensitive to bosutinib.
Adapted from Hochhaus et al.1
CONCLUSION
Ponatinib is the preferred option after resistance to one second-generation TKI, unless cardiovascular risk factors preclude its use, and is the only TKI with activity against the T315I mutation.

![EMJ Hematology 8 [Supplement 4] 2020 Feature Image](https://www.emjreviews.com/wp-content/uploads/2020/09/EMJ-Hematology-8-Supplement-4-2020-Feature-Image-940x563.jpg)





