Abstract
Immunotherapy has revolutionised the treatment of haematologic malignancies. Patients with relapsed/refractory non-Hodgkin’s lymphoma have poor response rates and short survival times when conventional cytotoxic chemotherapies are used. Immunotherapy offers a novel way to harness the host immune system to target malignant cells in patients whose disease may no longer respond to cytotoxic therapy. The increased and refined use of immunotherapy in this patient population has recently shown promise in a group with previously poor outcomes. In this paper, the authors describe the available data for immunotherapy use in non-Hodgkin’s lymphoma, including checkpoint inhibition, T cell engager antibodies, and adoptive immunotherapy with chimeric antigen receptor T cell therapy.
INTRODUCTION
While the long-term overall survival (OS) rates in the front-line setting for non-Hodgkin’s lymphoma (NHL) are upwards of 60–70%, a number of these patients will ultimately relapse and require further therapy.1 Among this relapsed/refractory (r/r) population, outcomes remain poor with standard cytotoxic immunochemotherapy. According to the SCHOLAR1 study, a large, patient-level pooled retrospective study of patients with refractory diffuse large B cell lymphoma (DLBCL), overall response rates (ORR) to the next line of therapy are 26%, with a median OS of 6.3 months. For this reason, earlier and more frequent consideration of novel therapies is warranted for these patients.
The last decade has shown a shift in the investigational and therapeutic arenas for NHL toward the use of immunotherapy. Under normal circumstances, the host immune system should recognise ‘self’ versus ‘non-self’ and aid in cancer prevention; however, tumours progress via circumvention of this safety mechanism with alterations in expression of surface antigens and evasion of the immune system via T cell exhaustion.2,3 Some of the earliest evidence that manipulation of the immune system effectively combats cancer came in the form of allogeneic stem cell transplantation.4 This treatment modality depends on the ‘graft versus tumour’ effect. The strength of a graft versus lymphoma effect is, however, unclear because data regarding the role for allogeneic transplant in lymphoma are inconsistent.5-8
In the 1990s and early 2000s, the benefit of antibody therapy became apparent with the resulting widespread adoption of rituximab for NHL.9 The addition of rituximab to cytotoxic chemotherapy with cyclophosphamide, vincristine, doxorubicin, and prednisone (CHOP) resulted in higher rates of complete response (76% versus 63%), as well as a statistically significant reduction in the risk of treatment failure (hazard ratio [HR]: 0.58) and death (HR: 0.64) in the elderly population. The MiNT study10 then demonstrated the added benefit of rituximab to CHOP in younger patients, with an improvement in 6-year event-free survival of 74.3% versus 55.8% among patients with and without rituximab, respectively. The success with rituximab marked the beginning of the use of antibodies targeted against various extracellular targets, such as radioimmunoconjugates and chemoimmunoconjugates in lymphoid malignancies.
Research in the field of oncology has seen a growth in our ability to harness the host immune system to target malignant cells in a more sophisticated and effective way. This review will discuss the existing data for novel immunotherapy in NHL, including immune checkpoint inhibition (CPI), T cell engaging antibodies (TCE), and chimeric antigen receptor T cell (CAR-T) therapies.
IMMUNE CHECKPOINT INHIBITION
The ability to redirect the host immune system against tumour cells has advanced our ability to treat malignancy. Monoclonal antibodies that block T cell inhibitory signals can revive the existing natural antitumour response in the host. Currently, six different U.S. Food and Drug Administration (FDA)-approved therapies exist in this realm: ipilimumab (anti-CTLA4), nivolumab and pembrolizumab (anti-PD1), and atezolizumab, avelumab, and durvalumab (anti-PDL1). While more data exist in solid malignancies, concurrent investigations in lymphoid malignancies have shown promise as well.
Both ipilimumab and anti-PD1 antibodies have been tested among NHL patients.11-14 A Phase I trial of ipilimumab in 18 patients with r/r NHL (follicular lymphoma [FL, n=14], DLBCL [n=3], and mantle cell lymphoma [MCL, n=1]) showed two objective responses.11 One patient with DLBCL had an ongoing complete response (CR) at >31 months and one patient with FL had a partial response (PR) lasting 19 months following ipilimumab. The most common adverse events with CTLA-4 inhibition in this study included diarrhoea, fatigue, abdominal pain, thrombocytopenia, and anaemia.
Lymphoma response rates to anti-PD1 therapy are more encouraging than those seen with ipilimumab. The efficacy of CPI in classical Hodgkin’s lymphoma (HL) is due to genetic alterations in 9p24 and subsequent overexpression of PDL1 on >90% of Reed–Sternberg cells.15 Although PDL1 is not as commonly expressed in NHL as in HL, there are subsets of patients who are specifically sensitive to CPI because of an overexpression of the PDL1 or PDL2 ligand. Primary mediastinal B cell lymphoma (PMBL) shares both histologic and genetic characteristics with classical HL, including genetic alterations in 9p24, which make it distinct from DLBCL. One group found that immunohistochemical stains showed PDL2 overexpression in 72% of PMBL biopsy specimens compared to only 3% of DLBCL specimens.16 Additionally, copy number gains and translocations of 9p24 commonly appear in both primary central nervous system (CNS) lymphoma and testicular lymphoma.17 Chronic infection with Epstein–Barr virus infection also leads to increased PDL1 expression on tumour cells.18
For these reasons, investigators have explored CPI in patients with PMBL, testicular lymphomas, and primary CNS lymphomas: subtypes of NHL known to have increased PDL1 expression. The KEYNOTE-013 trial14 examined the antitumour activity of pembrolizumab in 18 patients with heavily pretreated r/r PMBL and found an ORR of 41%, with stable disease in an additional 35% of patients. Of note, median duration of response was not reached at the median follow-up of 11.3 months. This trial was followed by the extended, multicentre, Phase II study of pembrolizumab in 49 patients with r/r PMBL (KEYNOTE-170).19 Intermediate follow-up data from KEYNOTE-170 presented in 2017 again showed an ORR of 41% with a 14% CR rate. Also in 2017, Nayak et al.13 published their experience with four patients with r/r primary CNS lymphoma and one patient with CNS relapse of primary testicular lymphoma treated with nivolumab. All five patients in this group had clinical and radiographic response to PD1 blockade, and three patients had progression-free survival lasting 13–17 months. The Checkmate-139 study20 is a Phase II study of nivolumab in patients with r/r DLBCL patients that is currently ongoing and should be very informative regarding the efficacy of PD1 blockade in relapsed DLBCL.
Anti-PD1 antibodies have also been investigated in NHL patients without 9p24 genetic alterations. In 2016, Lesokhin et al.12 published a Phase Ib study of nivolumab in 81 patients with r/r lymphoid malignancies. This cohort included FL (n=10), DLBCL (n=11), and other B cell lymphomas (n=10) with a median of three prior systemic therapies. In patients with FL and DLBCL, ORR were 40% and 36%, respectively. Among all patients with B cell lymphoma, median progression-free survival was 23 weeks (range: 7–44 weeks).
Data suggest that CPI may also have an effect through its interaction with PD1-expressing tumour infiltrating lymphocytes in malignancies in which the tumour cells do not overexpress these ligands themselves. For example, in FL, PD1+ cells, both tumour infiltrating lymphocytes and follicular helper T cells, comprise the tumour microenvironment.21 For this reason, FL is thought to be an extremely immunosensitive disease. Although results from single agent CPI in FL have not been as successful as the experience with HL, combination therapy with CPI and anti-CD20 antibodies seems to have a promising synergistic effect.
The MD Anderson Cancer Centre (MDACC) published their results of an open-label, non-randomised study of pidilizumab and rituximab in r/r FL.22 This study included 32 patients with previously treated FL and found an ORR of 66%, with CR in 52% and PR in 14% of patients at a median follow-up time of 15.4 months. No Grade 3 or 4 autoimmune adverse events were seen with this combination therapy. The combination of atezolizumab and obinutuzumab also showed favourable results, with an ORR of 57%.23 Pembrolizumab and rituximab combination therapy has shown an ORR of 80% among patients with r/r FL.24
Checkpoint Inhibitor Toxicity
In general, the side effect profile of immune checkpoint inhibitor therapy reflects the mechanism of action of these treatments via amplification of the patient’s immune response and includes autoimmune pneumonitis, hepatitis, thyroiditis, colitis, and hypophysitis. While safety data for these therapies are more robust within the realm of solid malignancies, available data regarding the toxicities among NHL patients mimic that of patients with solid malignancies.
The Phase I trial of nivolumab in solid malignancies showed an adverse event rate of 41%, with 6% of these Grade 3 or higher.25 Among the studies described above in NHL patients, similar results are seen. For example, in the Phase Ib study12 of nivolumab in r/r NHL, 34% of patients experienced immune-mediated adverse effects. Eleven percent of patients experienced pneumonitis with 4% Grade 3 or higher, including one death. This study also showed a 9% rate of pruritus and rash, 7% rate of diarrhoea, and 17% rate of fatigue. Endocrine toxicities include thyroid abnormalities seen in up to 10% of patients with solid malignancies and, less commonly, hypophysitis in about 1%.26 Given the wide range of immune-mediated toxicities, a comprehensive review of systems must be performed regularly along with basic laboratory and thyroid function testing with a low threshold to send chest imaging or additional laboratory tests to assess for autoimmune activity.
Of note, CTLA4 inhibition carries a higher risk of severe immune-mediated colitis not seen as commonly with PD1 inhibitors. The Phase I study11 of ipilimumab in NHL patients cited a 28% incidence of Grade 3 or higher colitis. Signs or symptoms suggestive of autoimmune colitis require prompt intervention with corticosteroids, with rapid escalation to infliximab for persistent symptoms.
Pembrolizumab recently received FDA approval for r/r PMBL after two or more therapies. While data show that CPI is safe and effective in patients with r/r NHL, the published literature is composed of small sample sizes and, as such, should be interpreted with caution. Larger studies moving forward will be critical to identify the role for CPI in NHL treatment and to investigate whether combinations with other treatment modalities are of additional benefit.
T CELL ENGAGER ANTIBODIES
TCE are another treatment modality that manipulates and invigorates the host immune response to tumour cells. Immunoglobulin fragments that can recognise two distinct epitopes transiently bring the host T cell face-to-face with a tumour cell. As with CPI, the autologous T cells are subsequently stimulated to kill tumour cells that express a given antigen.
Blinatumomab is one such TCE that recognises both CD19 on B cells and CD3 on T cells. This therapy has shown promise in B cell acute lymphoblastic leukaemia (ALL) and has been approved based on a study showing improved efficacy compared to salvage chemotherapy among heavily pre-treated B cell ALL patients.27 Concurrent studies have investigated the potential role for blinatumomab in NHL. In 2016, Goebeler et al.28 published the results of a Phase I study of blinatumomab among patients with r/r NHL. As in ALL, the short half-life of blinatumomab necessitates that this drug be given via continuous infusion over 28 days with dose-limiting side effects of neurotoxicity. Seventy-six patients with heavily pretreated NHL (FL: n=28; MCL: n=24; and DLBCL: n=14) were included for analysis and, of patients treated at 60 μg/m2/day, ORR was 69% across all subtypes and 55% among DLBCL patients with a median duration of response of 404 days. Neurologic events occurred in a dose-dependent manner, with 22% Grade 3 or higher events and 71% all Grade events among evaluable patients, with the most common events of headache (36%), tremor (18%), dizziness (15%), and aphasia (12%).
Viardot et al.29 later published a Phase II study of 21 patients with r/r DLBCL after a median of three prior lines of therapy. Overall response rate after one cycle of blinatumomab was 43% with a CR rate of 19%. Again, Grade 3 neurologic events were seen in a significant portion of patients, with encephalopathy and aphasia each seen in 9% of the patients.
As is shown in the aforementioned studies, blinatumomab carries promising efficacy in a heavily pretreated population of patients with aggressive NHL, although the data are not robust. Blinatumomab poses a significant risk for serious neurologic toxicity, which necessitates slow uptitration of the drug in the inpatient setting when initiating therapy. Additional TCE therapies under investigation include REGN1979 and FBTA05, which target CD20 and CD3, with the hopes of mitigating the neurotoxicity associated with anti-CD19 antibodies.30,31
CHIMERIC ANTIGEN RECEPTOR T CELLS
In the last few years, the genetic engineering of autologous T cells has been refined and broadened to commercial use in both B cell ALL and NHL. CAR-T are autologous T cells that are genetically modified to express a chimeric antigen receptor that consists of a transmembrane protein with an extracellular antigen recognition domain, a transmembrane hinge, and an intracellular signalling domain for T cell activation. Improvement in therapeutic efficacy has resulted from enhancement of both the costimulatory signals and the host conditioning prior to T cell infusion.
CAR therapy in haematologic malignancies was first described by Till et al.32 in 2008. However, without costimulatory molecules, first-generation CAR did not persist. It soon became evident that activation via costimulatory molecules was required for proliferation and persistence of the autologous T cells upon reinfusion.33,34 In later generation CAR, both CD28 and 4-1BB are commonly used. Potentially prolonged T cell survival is associated with 4-1BB when compared with CD28. Other costimulatory molecules, including CD27, OX40, and ICOS, are used less frequently.34,35
Further improvement in CAR efficacy came with lymphodepletion prior to T cell infusion. While the mechanism of improved disease response is not entirely understood, Maus et al.,34 among others, postulated that lymphodepletion with chemotherapy or radiation therapy may improve results by reducing regulatory T cells and decreasing disease burden prior to T cell engraftment. Additionally, the use of conditioning chemotherapy may increase the number of endogenous cytokines available for T cell engraftment by removing ‘cytokine sinks’ or other competing elements of the immune system.
Multiple centres have investigated the use of CD19-specific CAR-T cells in NHL, culminating in the first FDA-approved CAR-T products for r/r NHL patients. In 2015, Kochenderfer et al.36 first published their experience with anti-CD19 CAR-T cells in patients with chemotherapy-refractory DLBCL. This group from the National Cancer Institute (NCI) treated 15 patients with r/r NHL (DLBCL: n=9; chronic lymphocytic leukaemia: n=4; and indolent lymphoma: n=2) with fludarabine and high-dose cyclophosphamide prior to T cell infusion. Results showed 8 CR with durations between 9 and 22 months. This group later published their results with lower-dose conditioning chemotherapy for 22 patients with r/r NHL (DLBCL: n=19; FL: n=2; and MCL: n=1) in which patients received fludarabine 30 mg/m2 and cyclophosphamide 300 mg/m2 or 500 mg/m2 on Days -5 to -3 prior to infusion.37 Results showed an ORR of 73% and 55% CR rate, with duration of response ranging from 7–24 months. While the median duration of CR was 12.5 months, no PR or stable disease were ongoing at the time of analysis. Interestingly, high peak IL-15 levels were statistically significantly associated with higher peak blood CAR cells and remission rates compared to patients who did not achieve high IL-15 levels.
In 2017, the results from the ZUMA-1 clinical trial38 led to the approval of the first CAR-T product for r/r NHL. Neelapu et al.38 published their results of a Phase II trial of axicabtagene ciloleucel (Axi-cel, marketed as Yescarta® [Kite Pharma Inc., Los Angeles, California, USA]) in 111 patients with r/r DLBCL, PMBL, and transformed FL. This product uses the CD28 intracellular costimulatory domain and had previously shown promising results in a Phase I study.39 The ORR was 82% with a 54% CR rate and, at the median follow-up of 15.4 months, 42% of patients had an ongoing response, with 40% still in CR. Median duration of response among patients with CR was not reached in comparison to a median of 1.9 months among patients with a PR. OS at 18 months was 52%. These encouraging results led to the FDA approval of axicabtagene ciloleucel for the treatment of r/r DLBCL.
The University of Pennsylvania group published results with another anti-CD19 CAR-T product (CTL019) in 28 patients with r/r DLBCL or FL in 2017.40 Unlike the axicabtagene ciloleucel product, CTL019 uses a 4-1BB costimulatory domain. Lymphodepleting regimens in this study were chosen by the investigator and included cyclophosphamide, radiation, bendamustine, and modified EPOCH (doxorubicin, etoposide, cyclophosphamide without vincristine/prednisone). The ORR was 64% with a CR rate of 43% among patients with DLBCL and 71% in patients with FL, with responses maintained among 86% and 89% of patients with DLBCL and FL, respectively, at 28.6 months. Median duration of response was not reached. As a result, the FDA recently approved tisagenlecleucel (Kymriah® [Novartis, Basel, Switzerland]) for use in patients with r/r DLBCL.
Currently, axicabtagene ciloleucel and tisagenlecleucel are both approved for use in patients with r/r NHL, including DLBCL, DLBCL not otherwise specified, PMBL, high grade B cell lymphoma, and DBLCL arising from FL, after two or more lines of systemic therapy. Recommended dosage is 2×106–2×108/kg of CAR-positive viable T cells for axicabtagene ciloleucel and 0.6–6×108 /kg for tisagenlecleucel. Cyclophosphamide and fludarabine for 3 days prior to infusion is recommended as lymphodepletion therapy. It should be noted that T cell therapy complicates the assessment of response within the first 30 days and, thus, evaluation is needed at 3 months and longer to accurately measure treatment response.
Chimeric Antigen Receptor T Cell Toxicities
Given the recent commercial availability of these products, it is important to recognise the common side effects and management of adverse events related to adoptive immunotherapy. Cytokine release syndrome (CRS) has proven to be the most common, severe, and potentially life-threatening toxicity associated with CAR-T therapies.41 CRS typically presents within days of T cell infusion with median time to onset of 2 days (range: 1–12) for axicabtagene ciloleucel and slightly longer at 3 days (range: 1–51 days) for tisagenlecleucel, and consists of a clinical syndrome of fever (78%), hypotension (41%), hypoxia (22%), and tachycardia (28%).38,42,43 High levels of inflammatory cytokines, including IL-6 and IFN-γ, and elevated laboratory markers of inflammation, including ferritin, C-reactive protein, and hypofibrinogenaemia, have been associated with severe CRS.44,45
Severity of CRS, based on a grading scale of 1–3, dictates the management approach (Table 1).46 In mild CRS, expert opinion suggests supportive care with intravenous fluids, supplemental oxygen, and close monitoring for clinical decompensation. In cases of severe CRS with refractory shock or hypoxaemia, anti-IL-6 therapy with tocilizumab has proven efficacy, with benefit seen usually within hours of therapy.47 The FDA label mandates that two doses of tocilizumab are available for each dose of CAR cells prior to infusion. Of note, different grading scales between the different CAR products makes comparing toxicities between these products difficult.
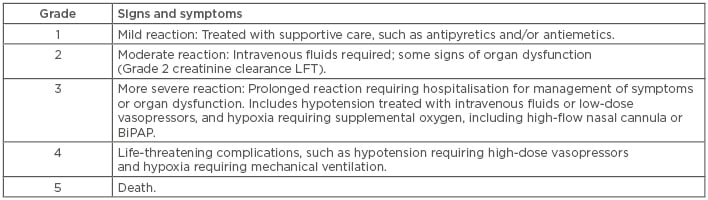
Table 1: Cytokine release syndrome: Penn Grading Scale.
BiPAP: Bilevel positive airway pressure; CRS: cytokine release syndrome; LFT: liver function test.
In addition to CRS, neurologic toxicities appear in a significant portion of patients after T cell infusion (Table 2). Neurologic events, including encephalopathy (57%), headache (44%), tremor (31%), dizziness (21%), and aphasia (18%), occurred in 87% of patients, with a median time to onset of 4 days.38 The FDA recommends treatment for Grade 2 or higher neurologic toxicity with tocilizumab, in the presence of concurrent CRS, or with intravenous corticosteroids in the absence of concurrent CRS. Providers should also consider prophylactic antiepileptics for any Grade ≥2 neurologic toxicities. Toxicity profiles and rates of events are similar between the two commercially available CAR products.
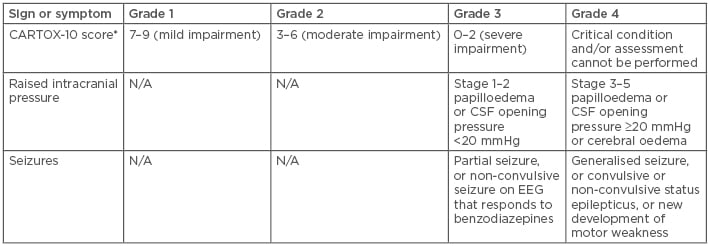
Table 2: Chimeric antigen receptor T cells encephalopathy syndrome grading.
*CARTOX-10 score: Five points for being able to name the year, month, city, hospital, and president or prime minister of their home country (one point per answer). Three points for being able to name three nearby objects (one point per answer). One point for writing a standard sentence and one point for counting backwards from 100 in tens.
CSF: cerebrospinal fluid; EEG: electroencephalogram; N/A: not applicable.
CONCLUSION
In summary, immunotherapy in the r/r NHL patient population has shown promising progress in the last several years. As this patient group has previously had an extremely poor prognosis with relapsed disease after failing front-line therapy, the published literature described above offers hope for patients with typically poor outcomes. CPI and TCE, along with adoptive immunotherapy and CAR-T cells, result in high remission rates and prolonged responses among this heavily pretreated population. Currently, the appropriate timing for these novel agents in the sequence of lymphoma treatments remains unknown. With longer-term follow-up data that enables us to compare outcomes of adoptive immunotherapy to those of more well established treatment modalities, such as autologous stem cell transplantation for relapsed lymphoma, we will perhaps be able to better identify when to use these novel therapies in the treatment of these patients.







