Abstract
May-Thurner syndrome is an underappreciated differential in the context of deep venous thrombosis. It is a symptomatic congenital abnormality resulting from compression of the left common iliac vein, sometimes also referred to as iliac vein compression syndrome in the literature. This case reports the presentation of a female with acute flank pain to the emergency department following a recent laparotomy for a complicated tubo-ovarian abscess. She was subsequently diagnosed with a sub-acute thrombus extending from the left common femoral vein into the left inferior vena cava, as well as new bilateral pulmonary emboli. As the patient had relative contraindications to thrombolysis, a shared decision was made with the vascular team to opt for conservative management only. In this instance, this included long-term oral anticoagulant therapy and compression stockings. This case asserts that clinicians should maintain a high index of suspicion for May-Thurner syndrome in the context of extensive left-sided thrombus formation.
Key Points
1. Clinicians should maintain a high index of suspicion for May-Thurner syndrome and consider it in the diagnostic work-up of at-risk patient groups.
2. Shared decision-making with patients is integral in deciding whether to deviate from gold-standard treatment where relative contraindications/cautions are present.
3. The therapeutic management of chronic venous thrombi resulting from May-Thurner syndrome is not well defined in current literature.
BACKGROUND
May-Thurner syndrome (MTS) is defined as symptomatic compression of the left common iliac vein by the overlying right common iliac artery against the lumbar vertebrae.1 The compression of this specific element of the left-sided venous system was first observed to be a precipitating factor in deep venous thrombosis (DVT) formation by Virchow in 1851, who identified a link between this anatomical variant and left-sided lower limb clot formation.2 However, it was May and Thurner’s prominent 1957 study that exposed the underlying pathophysiology.3 Using 430 unselected cadavers, they revealed that intraluminal fibrous bands (termed ‘spurs’) tended to form within the left iliac vein if it was compressed, a result of chronic irritation of the venous endothelium by arterial pulsation.3 In addition, the results of this study also drew evidence for the prevalence of this abnormality. Although a small sample size was used, compression plus ‘spur’ formation was present in approximately 22% of the cadavers used.3 This has been corroborated by more recent retrospective radiological studies of patients with confirmed left-sided DVT. For example, one 2004 study, using spiral CT venography, found that 61% of patients with confirmed left-sided DVT had evidence of left iliac vein compression.4 Despite these studies, the overall prevalence of this anatomical variant remains challenging to ascertain as it is thought to be clinically silent in most individuals.5
The compression of the left iliac venous system can only be termed MTS in those who are symptomatic, with a currently estimated prevalence of 14–32% in the general population.2 Many patients may present with signs consistent with venous hypertension, including left lower limb swelling or claudication. However, a significant proportion only present once they have developed signs consistent with left lower limb thrombosis or a potentially life-threatening pulmonary embolus. A significant sex difference has been noted amongst these symptomatic patients, with one 2018 systematic review estimating the female-to-male ratio at 2:1.6 Notably, this review also found that females were more likely to have a pulmonary embolus on presentation.6 Overall, the demographic most at risk of MTS has been identified as females aged 20–40 years.7
CASE PRESENTATION
A female in her 40s presented to the emergency department with a 6-hour history of paraesthesia and unilateral left-sided calf swelling. They subsequently developed severe left-sided flank pain 2-hours after the onset of swelling. The pain was severe enough to warrant multiple doses of IV morphine. Their significant past medical history included multiple recent admissions with left-sided tubo-ovarian abscess, culminating in an emergency laparotomy, with a 1-day intensive therapy unit admission post-surgery. Since discharge, they acknowledged a significant decline in her mobility levels. The patient was also known to be a heavy smoker.
On initial examination, the patient had mild hypotension (91/60) and low-grade pyrexia (37.7 °C) but was otherwise haemodynamically stable. On palpation of the abdomen, there was left-sided renal angle tenderness without evidence of peritonism. The left calf was also visibly swollen, with pitting oedema to mid-shin, but was non-tender on palpation. The rest of the examination was unremarkable.
Laboratory studies requested on admission revealed a leucocytosis (18.7) with neutrophilia (12.7). Urine dipstick was negative. Initial coagulation studies, including D-dimer, had haemolysed and were sent for a repeat. An interim CT arterial portography considering her complex surgical history was requested and subsequently completed. This revealed a ‘highly suspicious acute/subacute DVT on a background of MTS with a tongue of thrombus extending from left common femoral vein into lower inferior vena cava’. There was also a ‘highly suspicious right lower lobe pulmonary embolism (PE)’. (Figure 1, Figure 2) D-dimer was also reported shortly after at 3,969 and other coagulation studies were unremarkable. The patient was subsequently admitted under the medical team and treatment dose anticoagulation was initiated with tinzaparin.
A dedicated CT pulmonary angiogram was requested following admission. This revealed ‘segmental and subsegmental PEs affecting the right lower lobe with evidence of wedge infarction in the right lower lobe’. (Figure 3) ‘Subsegmental PEs affecting the lower lobe’ were also identified. The patient was discussed with the vascular on-call registrar, who advised that there was no sufficient indication for urgent catheter-directed thrombolysis as an element of chronicity had been identified. Furthermore, their recent abdominal surgery was also a relative contraindication to any thrombolytic procedure. A thrombophilia screen was also sent at this point and was subsequently reported several days later as unremarkable. On discharge, follow-up was arranged in vascular and haemophilia clinics. On outpatient vascular review 5 days post-discharge, a shared decision was made with the patient to opt for conservative management only. This entailed continuation of anticoagulation, albeit with a switch made to rivaroxaban, and orthotics assessment for an above-knee stocking. They were also advised on smoking cessation and signposted to relevant resources.
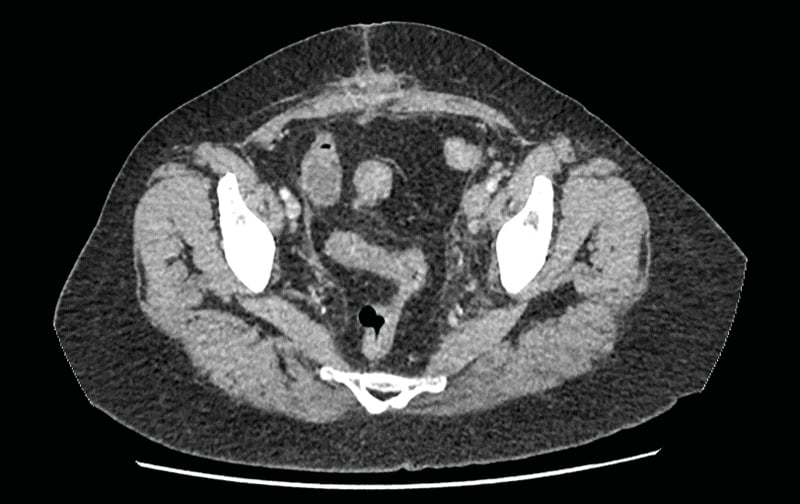
Figure 1: Axial section from the CT abdomen pelvis with contrast demonstrating significant distension and filling defect in the left common iliac vein.
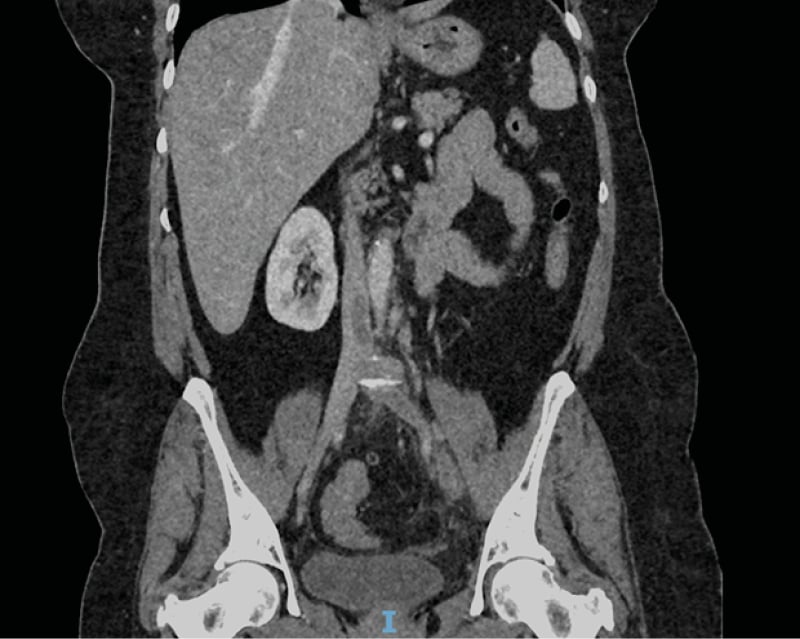
Figure 2: Coronal section from the CT abdomen pelvis with contrast demonstrating tongue of thrombus at the inferior part of the inferior vena cava.
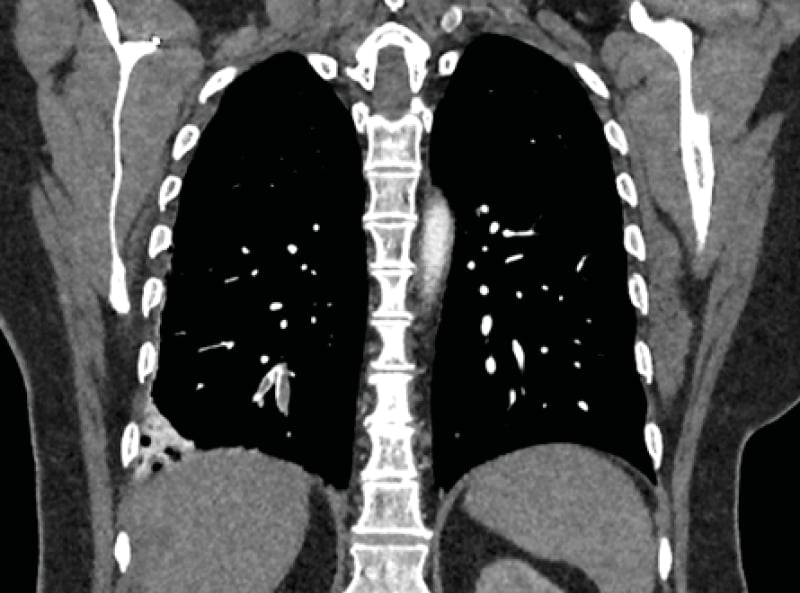
Figure 3: Coronal section from the CT pulmonary angiogram demonstrating a right lower lobe pulmonary embolism with evidence of wedge infarction.
DISCUSSION
Current literature suggests that MTS as a contributing factor to DVT formation is significantly underdiagnosed, particularly given the estimated population prevalence of iliac vein compression, as pointed to by cadaveric and radiographic studies.7,8 This is likely secondary to a combination of factors. Firstly, Doppler ultrasound is currently the most common modality used in emergency departments to diagnose acute DVT. However, it is often unable to adequately visualise the deep iliac vessels and therefore identify compression of the left iliac vein.9 Due to this, the gold standard for MTS diagnosis is venography with intravascular ultrasound, which has both a high sensitivity and specificity but is not necessarily a practical investigation in the acute setting.2 Secondly, for a patient, if a DVT is diagnosed and a transient risk factor is identified, further investigations are often not considered to identify any pre-existing vulnerabilities in the patient’s anatomy. This is significant as the population group most affected, younger females, are in a key demographic for additional risk factors such as the combined oral contraceptive pill and pregnancy. Imaging to identify MTS could therefore allow for further risk stratification, as part of the diagnostic work-up, once DVT is confirmed.
For patients recognised to have MTS without thrombotic complications, early management strategies may include compression stockings and systematic anticoagulation. However, this is not considered sufficient long-term due to failure to address the underlying anatomical precipitant.10 The mainstay of interventional therapy for MTS therefore consists of iliofemoral stent insertion.11 However, clinical outcomes, including stent failure or long-term patency, have been shown to be less favourable for patients with thrombotic sequelae of MTS versus those without.12 Therefore, there is a growing body of research that suggests catheter-directed thrombolysis is preferable for patients with both MTS and DVT formation, with further long-term anticoagulation therapy post-procedure.13 However, in this specific case, management decisions were complicated by an element of chronicity identified by CT imaging, for which thrombolysis is not as useful.14 This was potentially linked to the recent removal of a tubo-ovarian abscess that was likely contributing to further external compression of the left pelvic veins. Furthermore, the patient’s recent open laparotomy within the previous month was also a relative contraindication. Therefore, the case also highlights the importance of shared decision-making between the multidisciplinary team and the patient. For this patient, once the above complicating factors were outlined to her, she opted for conservative management with systematic anticoagulation alone. Furthermore, regarding the duration of systematic anticoagulation for patients with MTS, there is no standardised consensus. This is irrespective of whether interventional therapy has been pursued or not, with one small case series reporting variability in therapy from 6 months to life-long.15 There is also no significant evidence base determining the choice of oral anticoagulation used.2
CONCLUSION
There are a growing number of case reports describing extensive thromboses with MTS acknowledged as a contributing factor.16-18 This report serves as an additional account to strengthen awareness of this anatomical syndrome and its consideration in the diagnostic work-up of select patient groups. Furthermore, this case also highlights the importance of shared decision-making with the patient when management options are not well defined, such as in the case of a sub-acute thrombus.






