Abstract
Since the late 1990s, when the first tyrosine kinase inhibitor (TKI) imatinib was introduced as a front-line treatment for chronic myeloid leukaemia, the disease’s course and prognosis has dramatically changed. The development of second-line and further-line more potent generations of TKI has further improved disease control and patients’ quality of life; however, during this time, many questions such as the duration of treatment, the depth of response, fertility, pregnancy, and family planning, have been raised. Recent prospective and retrospective discontinuation trials for TKI have shown encouraging results regarding the cessation of TKI treatment and maintaining complete molecular response. The authors report three cases of female patients diagnosed with chronic phase chronic myeloid leukaemia who achieved a long-term deep molecular response; had planned management during pregnancy, including regular molecular monitoring with or without INF-α; and all delivered healthy babies.
INTRODUCTION
BCR-ABL1 positive chronic myeloid leukaemia (CML) is a myeloproliferative neoplasm in which granulocytes are the major proliferative component. BCR-ABL1 CML positive arises in haematopoietic stem cells and is characterised by the chromosomal translocation t(9;22)(q34.1;q11.2), which results in the formation of the Philadelphia chromosome, which contains the BCR-ABL1 fusion gene.1 This fusion gene produces a unique oncoprotein named BCR-ABL, which is an active tyrosine kinase. At the very end of the 1990s, CML became a targeted therapy success in oncohaematology due to the introduction of the first tyrosine kinase inhibitor (TKI), imatinib mesylate, which specifically binds to the ATP binding site of the BCR-ABL kinase and inhibits it.2 Over the following years, second and third-generation TKI were approved as part of the armamentarium for the treatment of CML.
Patients diagnosed in the chronic phase can achieve excellent disease control and therefore expect many years of good quality of life; furthermore, patients who achieve an optimal response can reach a life expectancy similar to a non-leukaemic population of the same age.3
Globally, the median age at diagnosis reported in the literature for patients with CML is about 55–60 years.2 In Bulgaria, the median age newly diagnosed CML patients is 51.1+14.5 years old, based on data from 499 patients (unpublished data). A number of issues have been raised by patients to physicians regarding contraception, teratogenicity, fertility, and pregnancy. Currently, there is no evidence-based consensus for the management of CML in pregnancy; therefore, clinical observations have become very important. Most of these observations are derived from small case series or case reports.
THE AUTHORS’ EXPERIENCE: THREE PREGNANT FEMALE PATIENTS WITH CHRONIC PHASE CHRONIC MYELOID LEUKAEMIA
Between April 2014 and January 2018, three planned pregnancies were managed by the CML team at the Medical University of Plovdiv, Bulgaria. Details of the patients can be seen in Table 1.
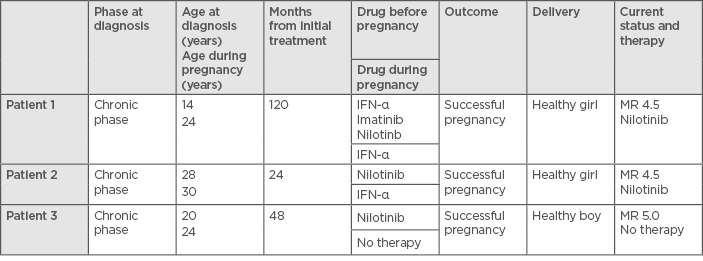
Table 1: Characteristics of the three pregnant female patients with chronic phase chronic myeloid leukaemia.
MR: molecular response.
To take part in the planned pregnancy management, several factors were required: patient at the chronic phase of the disease at the time when the diagnosis was established, no history of accelerated or blast phase CML, on therapy with TKI, and stable molecular response (MR: 4; BCR-ABL1 <0.01% IU) for >2 years, as documented on at least four tests performed at least 3 months apart. During the pregnancy, after stopping the TKI, the patients were treated with INF-α, based upon the authors’ previous experience in two patients who were treated with INF-α during their pregnancies in the pre-TKI era and gave birth to two healthy children. BCR-ABL1 transcripts were measured every second month during the pregnancy. The authors have not experienced any unsuccessful or complicated pregnancies in patients treated with TKI.
Patient One
The patient was diagnosed with chronic phase CML (CP-CML) at the age of 14 years (Sokal score: low risk; Hasford score: low risk) and began therapy with IFN-α for 48 months, then switched to imatinib. After 24 months therapy with imatinib, the patient was switched onto nilotinib, a second-generation TKI, due to an adverse event (skin changes). The patient received nilotinib for 48 months before ceasing the therapy due to planned pregnancy. From 2012–2014, her BCR-ABL1 transcript level was monitored every 3 months and the results showed a stable molecular response (MR) of 4.5. One year after the discontinuation of nilotinib therapy, the patient became pregnant. During the second trimester, a loss of the MR of 4.5 was detected and therapy with INF-α was started. A cytogenetic test for evaluation of the cytogenetic response was not carried out. No side-effects or complications while receiving INF-α therapy were registered. The patient delivered a healthy girl per vias naturales and the therapy with nilotinib was restarted soon after. The patient did not breastfeed the baby, and 3 months after the delivery, the patient regained a MR of 4.5.
Patient Two
The patient was diagnosed with CP-CML at the age of 28 years (Sokal score: low risk; Hasford score: low risk) and started first-line therapy with nilotinib. Twelve months after the treatment was started, the patient obtained a MR of 4.5. During the second year of the treatment, she was monitored every 3 months and the results showed a durable MR of 4.5. After 24 months of nilotinib therapy, the patient ceased the TKI therapy because of planned pregnancy. Fourteen months after stopping nilotinib, the patient became pregnant. During the pregnancy, she maintained a MR of 4.5. Pre-emptive treatment with INF-α was started at the end of the first lunar month and the patient remained on INF-α therapy until the delivery; no side-effects or complications were registered while the patient received INF-α therapy. The patient delivered a healthy female baby at gestational Week 39 via caesarean section. The patient did not breastfeed the baby. The child developed normally without any evidence of congenital malformation. The treatment with nilotinib was restarted soon after the delivery and, to date, the patient has maintained a MR equal to 4.5.
Patient Three
The patient was diagnosed with CP-CML at the age of 20 years (Sokal score: low risk; Hasford score: low risk) and started first-line therapy with nilotinib. Twelve months after the treatment was started, she obtained a MR of 5 and maintained the MR for the next 3 years. Treatment was stopped 4 years after starting TKI therapy because of the planned pregnancy. Fifteen months after stopping nilotinib, the patient became pregnant. During the pregnancy, she maintained a molecular response equal to a MR of 5 without any treatment and remained at that level until the delivery. She delivered a healthy male baby at gestational Week 40 via caesarean section and breastfed the baby. The child developed normally without any evidence of congenital malformation. Since the delivery, the patient has not restarted TKI therapy, her BCR-ABL1 transcript levels are monitored every 3 months, and she is still at a MR of 5.
Case Summary
In summary, a stable MR of 4.5 for 4 years is not always indicative that it is safe to discontinue TKI, as seen in Patient One. However, the patient regained a MR of 4.5 soon after restarting treatment with TKI. Treatment with IFN-α is safe and effective after discontinuation of TKI and during pregnancy.4-6 As some reports indicate, restarting treatment with imatinib from the second trimester during pregnancy in patients with a high risk of relapse is a feasible approach.7,8
DISCUSSION
During the last two decades, the clinical view and management of CML has changed dramatically. TKI have revolutionised the treatment of CML, leading to a substantial improvement in survival, disease control, and quality of life in patients with CML. Considering the fact that treatment with TKI nowadays is recommended to continue indefinitely as far as it is tolerable or the disease is under control, patients frequently raise the question of stopping the therapy.
The main causes for cessation, according to recently published case reports and series, were adverse events, including intolerance (50%), the patient’s choice (26%), the cost of drugs (12%), and pregnancy (12%).9-16 There is currently no official guideline regarding management of CML in cases of pregnancy. The data reported are from several prospective and retrospective clinical trials analysing the discontinuation of TKI in CP-CML.17-21 Based on the data from the multivariate analysis of these trials, there are some important risk factors that impact the maintenance of complete MR (CMR) after stopping the TKI therapy. A high Sokal score is a significant independent risk factor for relapse after cessation of TKI therapy. Factors associated with longer TKI free-remission include prior IFN therapy before TKI therapy, longer duration of CMR before discontinuation, and longer duration of imatinib mesylate use before discontinuation (>60 months). Additionally, achieving an early deep molecular response at 3 months is associated with a durable deep molecular response, as indicated by a recently published analysis.20-26
An independent algorithm was proposed by Milojkovic and Apperley8 for the management of CML patients diagnosed during pregnancy.They proposed leukapheresis during the 1st–3rd trimester, the frequency of which is determined by the need to maintain a leukocyte number <100×109/L and a platelet count <500×109/L. In the second and third trimester, INF-α is indicated as an option for treatment for patients having a suboptimal response to leukapheresis. Patients planning an elective pregnancy who have complete haematological response or a better response with TKI are recommended to collect oocytes for future assisted conception, stop TKI at the onset of their menstrual cycle, and start in vitro fertilisation medications 7 days after TKI discontinuation. TKI should be restarted after oocyte collection. Patients planning pregnancy with a stable major molecular response or better MR for 24 months can stop TKI at the onset of the menstrual cycle and should undergo reverse transcription quantitative (qRT-PCR) monitoring in addition to examination of peripheral blood during pregnancy.8
Supporting the idea of quiescent residual CML stem cells, qRT-PCR for BCR-ABL1 transcripts is a mandatory test for pregnant patients with a MR of 5. Disease monitoring during pregnancy, according to some published data, should include qRT-PCR every month if a CMR of 4.5 is not achieved and qRT-PCR every 2 months if the patient presents with CMR.8,27-30 In cases of a loss response, the risk to the mother and the baby should always be considered.
After delivery, all patients can breastfeed for the first 2–5 days postpartum to provide the baby with colostrum. The reason for this is that newborns have very immature digestive systems, and, as such, colostrum is needed to deliver nutrients in a very concentrated low-volume form. Due to its mild laxative effect, colostrum aids the passage of the baby’s first stool. It also helps to clear excess bilirubin. Colostrum contains immune cells and many antibodies, immune substances, and a series of cytokines and growth factor. Considering the few days of delay before treatment resumes, it might be important for the newborn to access breastfeeding. After delivery and sustaining a good molecular transcript level, treatment with TKI can be postponed to enable full breastfeeding, according to the haematologist’s judgment.31
Most of the published cases with good disease control at conception and optimal response while being on TKI stopped during the pregnancy and restarted after delivery confirm the possibility of a safe therapeutic management during pregnancy.32-34
CONCLUSION
In conclusion, it is very important to take into consideration that each case of CML in a pregnant patient should be treated as a separate case due to the influence of many factors on the pregnancy outcome, such as the biology of the disease, duration of the treatment, compliance with therapy, and response to the treatment. The willingness of the patient should always be considered and discussed. As a new generation of TKI is incorporated into the clinical practice, the percentage of patients achieving MR ≥4.5 will be constantly increasing. Continuing effort is needed to determine the optimal management of pregnant patients with CML.







