BACKGROUND
Bone marrow aspiration and biopsy (BMAB) is a painful procedure. The commonly adopted local infiltration anaesthesia (LIA) with lidocaine is unable to relieve the pain during the most uncomfortable phases, or the anticipatory anxiety related to pain recalled thereafter. As there are no formal guidelines for adding a sedoanalgesic premedication before beginning BMAB, many combinations have been adopted by several authors.1-8
AIMS
Our randomised and patient-blinded trial aimed to evaluate the efficacy and safety of opioid and benzodiazepine agent combination plus LIA in patients who underwent BMAB for haematological malignancies, which was the primary endpoint of the trial. The secondary endpoints were firstly, to define if patients who had undergone BMAB without LIA preferred sedoanalgesia and secondly, to demonstrate if sedoanalgesia could influence the quality of the biological specimen harvested.
METHODS
Patients were randomly assigned into two arms, receiving either placebo plus LIA (standard group: 48.6%) or oral fentanyl citrate 200 µg plus oral midazolam 5 mg in addition to LIA (combo group: 51.4%) during BMAB. Preprocedural anxiety and procedural pain were assessed according to the Numeric Rating Scale (0–10), dividing the time of the procedure into five intervals (T0, T1, T2a, T2b, and T3) and evaluating discomfort grade during each moment of the procedure in both groups. Cognitive function was measured before and 30 minutes after the procedure. Possible side effects were recorded, as well as the adequacy of tissue samples harvested. A telephone interview was performed 24 hours later. A total number of 116 patients were enrolled in the study. Nine patients did not meet the inclusion criteria and were excluded. Fifty-two patients were randomised and assigned to the standard group and 55 to the combo group (Figure 1).
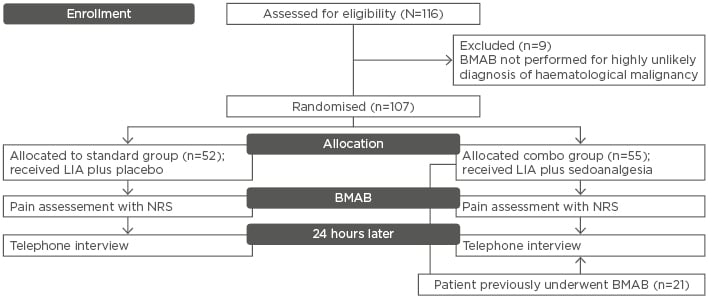
Figure 1: Flow chart of the trial.
BMAB: Bone marrow aspiration and biopsy; NRS: Numeric Rating Scale; LIA: local infiltration anaesthesia.
RESULTS
At T2b and T3 (corresponding to the biopsy time and time after the biopsy, respectively) there was a significantly lower (p<0.05) perception of pain in the patients who received sedoanalgesia (combo group) compared to those who did not (standard group). Moreover, 100% of the patients in the combo group who had previously undergone this procedure without premedication reported that they would prefer sedoanalgesia for the subsequent procedures, thus confirming the effectiveness of this combination in relieving anticipatory anxiety. Finally, the histological specimen was found to be high in quality, as defined by International Council for Standardization in Haematology (ICSH) standards.9
CONCLUSION
Administration of oral analgesia and anxiolysis is a safe and feasible option to be used in the outpatient setting; sedoanalgesia is very effective in reducing pain during the biopsy and it diminishes the anticipatory anxiety related to a painful procedure. Patients should have the option to choose between local anaesthesia alone or sedoanalgesia plus local anaesthesia.







