Meeting Summary
Despite the fact that the treatment armamentarium for inflammatory bowel diseases (IBD) is growing, unmet medical needs remain. These needs are driven, at least in part, by restricted access to biologics, which means that patients who would benefit from these agents will not receive them. This symposium explored approaches to improve IBD care, evaluating both the potential of novel therapies and the role of optimised treatment using the treat-to-target concept and careful evaluation of use of the right drug at the right time. The reality for clinicians is that selecting the best treatment needs to take into account the best medical option, patient preferences, and cost, which is one of the main barriers limiting access to biologic treatment. In this regard, biosimilars could serve the patient community by facilitating increased access, including use in early intervention to avoid disease progression. Education around biosimilars is essential to ensure patient acceptance of these agents and maximise the opportunity that they provide.
Introduction
Professor Walter Reinisch
Anti-TNF biologics are now well-established standard of care treatments that have significantly improved quality of life and reduced the need for hospitalisation and surgery for patients with IBD. At the same time, novel treatments and therapeutic approaches, which have the potential to further improve patient outcomes, continue to be investigated.1 While advances in therapy are almost invariably associated with increased costs, the availability of biosimilars offers the opportunity for cost savings, and the potential to increase access to treatment.2 Prof Reinisch highlighted how the accumulating evidence from recent trials, along with a better understanding of biosimilar development and regulatory approval, have helped to bring about a change in the perception of IBD specialists, such that they now prescribe biosimilars with increased confidence. This is acknowledged in the European Crohn’s and Colitis Organisation’s (ECCO) updated position statement on biosimilars.3 The symposium addressed advances both in novel treatments and in biosimilar developments, and for the latter considered the clinicians’ and patients’ perspectives, which are both important to maximise the potential benefits that can be achieved.
Preparing For the Next Era in the Management of Inflammatory Bowel Diseases
Professor Jean-Frédéric Colombel
Prof Colombel noted that the incidence of IBD continues to increase steadily in Western countries and more dramatically in newly industrialised countries, such as China and India.4 However, despite discussions around the best treatment options, cost remains a barrier to biologic therapies, which can lead to restricted access and suboptimal treatment strategies. A report by Siegel et al.5 highlighted real-world evidence from the USA indicating that, from January 2008–March 2016, only a small proportion of patients (<5%) received biologic therapeutics. In contrast, approximately 30% of IBD patients initially received treatment with 5-aminosalicylic acid (5-ASA), which is not approved for Crohn’s disease (CD) in the USA, and many continued to receive this agent during the treatment pathway.5 Three broad approaches were discussed to address unmet needs in IBD: improving current care, searching for a cure, and exploring prevention strategies (Table 1).

Table 1: Approaches to address unmet needs in inflammatory bowel disease treatment.
Early intervention with biologics, treat-to-target, and tight control of clinical symptoms and biomarkers are essential for optimising current IBD care. The importance of early intervention was highlighted in a recent study of 130 patients with CD: while bowel damage increased with disease duration, damage was reduced in patients who received anti-TNF therapy within the first 2 years of disease progression compared with those exposed later.6 A treat-to-target approach focusses on achieving remission or low disease activity using evidence-based treatment targets. This involves patients and clinicians agreeing strict definitions for treatment targets and working towards achieving them by adopting changes in therapy within distinct time frames.7 The current target is clinical remission of symptoms and mucosal healing.7 Tight control involves treatment decisions based on regular monitoring of intestinal inflammatory biomarkers, such as C-reactive protein (CRP) and faecal calprotectin, and clinical symptoms.8 The benefits of tight control of disease activity are illustrated by results from studies such as CALM,9 an open-label, randomised, controlled Phase III study of patients with active endoscopic CD. Adalimumab initiation, escalation, and de-escalation, driven by monitoring a combination of CRP and faecal calprotectin biomarkers, Crohn’s Disease Activity Index (CDAI), and prednisone use, led to improvements in the rate of mucosal healing and an absence of deep ulcerations 48 weeks after randomisation, compared with clinical management using escalation, based on CDAI and prednisone use alone.
While novel treatments have provided further benefits to patients, the efficacy of these drugs, such as ustekinumab, is beginning to plateau during maintenance treatment10 and there is a need to find alternative approaches. Promising new drugs to treat IBD include additional JAK inhibitors (such as tofacitinib, which is already approved for ulcerative colitis [UC] in Europe)11 and the sphingosine 1-phosphate receptor modulator class.12 There is also growing interest in novel approaches to combine biologics in IBD therapy, for example, using adalimumab in combination with vedolizumab,13 and the cost savings associated with biosimilar treatment may help support such an approach. Future treatment strategies are anticipated to include personalised medicine approaches and the increasing use of diagnostic and predictive biomarkers. For example, a recent publication by West et al.14 demonstrated that low pretreatment oncostatin M levels compared with higher levels of the protein in the mucosa were associated with improved complete mucosal healing following infliximab therapy in patients with IBD.
The concepts of ‘cure’ and ‘prevention’ in IBD were explored during the symposium. It was noted that understanding the mechanisms underlying IBD in an individual is crucial, and this includes the genetic, microbial, immunological, and metabolomic profiles and the clinical phenotype. Genetic analysis may be useful for identifying the causes of IBD and offering the patient appropriate treatment aimed at correcting the defective pathway. This was illustrated by the case of an early- onset IBD patient with a homozygous mutation in an IL-10 receptor (IL10R2) who had disease remission following allogeneic stem cell transplantation from a sibling with a normal IL10R2 gene.15 However, given the genetic complexities of IBD,15 a cure is unlikely to be reached in the near future. Consequently, effective disease prevention, through an improved understanding of the preclinical phase of disease, is essential. During this phase, environmental and genetic factors interact and result in initiation and propagation of disease followed by subclinical inflammation and tissue damage.16 Increasing evidence demonstrates that this preclinical period, in which immunological changes in inflammatory markers and antimicrobial antibodies can be detected, can occur years before IBD diagnosis.17 A serological tool has recently been developed, using microbial antibodies and proteomic markers, that can predict CD around 5 years before the first symptoms.18 Gaining further insight into this preclinical phase of IBD could pave the way for preventative strategies.
Prof Colombel concluded his presentation by summarising that IBD are progressive, complex, heterogeneous diseases, the importance of optimising current treatments, and how disease prediction and prevention are likely to be central for the future of IBD management.
Upcoming Biosimilars in the Spotlight: What to Consider When Selecting
Professor Walter Reinisch
The anti-TNF biologics infliximab and adalimumab are effective and established treatments for adult and paediatric CD and UC patients. Biosimilars of these agents are now available in Europe, adding to the range of potential treatment options (Table 2).11,19 Prof Reinisch provided an overview of the anti-TNF agents available and discussed some of the key factors that clinicians should consider when evaluating them for use in the clinic.
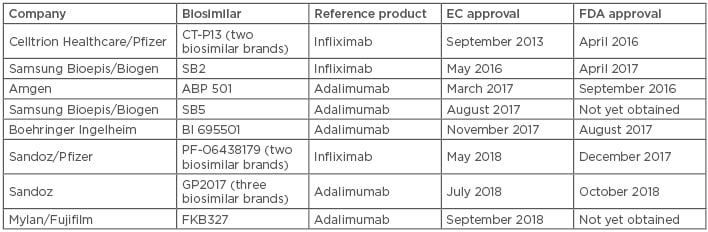
Table 2: Approved adalimumab and infliximab biosimilars in Europe and the USA (November 2018).11,19
EC: European Commission; FDA: U.S. Food and Drug Administration.
The totality of evidence is the data package generated for a biosimilar to demonstrate that it is equivalent to the reference product. This focusses on analytical and functional analyses of the biosimilar supported by data from clinical studies. For biosimilars, guidelines suggest that only one confirmatory trial is generally required, and clinical studies are designed to demonstrate that there are no clinically meaningful differences compared with the reference product rather than efficacy and safety per se. Biosimilar clinical studies are usually equivalence trials and the most sensitive patient populations and clinical endpoints should be identified to ensure that any differences between the biosimilar and reference product with respect to efficacy, safety, and immunogenicity can be attributed to product characteristics rather than patient and disease-related factors.20,21 Adalimumab biosimilars have primarily been evaluated in Phase III trials of moderate-to-severe plaque psoriasis (PsO) and/or rheumatoid arthritis (RA). In PsO, there is a relatively high placebo-adjusted response rate. This, combined with the fact that biologics are not usually administered with immunosuppressive therapy, facilitates the detection of small differences in efficacy, safety, and immunogenicity. However, assessment in RA does allow for comparison between biosimilar and reference product in combination with standard of care immunosuppressive agents.22 Evaluating biosimilars in IBD models is difficult because of the high inter-individual variability in pharmacokinetics and because surrogate markers, such as therapeutic drug monitoring, are required to assess systemic inflammation. Patients with IBD also display heightened immune responses that can lead to accelerated drug clearance relative to other populations.23 The use of biosimilars is, therefore, generally extrapolated to the IBD population at the time of licensing.
Data supporting switching from reference product to biosimilar are important to provide confidence in continued efficacy, safety, and immunogenicity. Because all biosimilars are unique, data on switching should be assessed for each agent. Switching data for the adalimumab biosimilar ABP 501 were discussed during the symposium as an example of the data that may be generated. Equivalence of ABP 501 and the adalimumab reference product has been demonstrated in terms of efficacy, safety, and immunogenicity in two Phase III, randomised controlled equivalence trials: one in moderate-to-severe PsO (N=350)24,25 and one in moderate-to-severe RA (N=526).26 In the 52-week Phase III PsO trial, a switch occurred at Week 16, with patients who were initially randomised to the adalimumab reference product arm being re-randomised to continue on the reference product or to receive ABP 501. Patients treated with ABP 501 and adalimumab reference product had similar clinical efficacy, safety, and immunogenicity profiles over the duration of the trial, including after the single switch.24,25 In RA, a 26-week Phase III parent study26 was followed by a 72-week, open-label extension study27 (i.e., total duration of 98 weeks), in which all eligible patients, including those initially receiving adalimumab reference product, could continue on ABP 501. In the open-label extension study, efficacy, safety, and immunogenicity were comparable to those seen in the parent study.27-29 The formulation may be an important consideration when evaluating different adalimumab biosimilars, as injection-site pain can impact patient acceptance of treatment. It was noted that patients receiving ABP 501, which is citrate-free, reported lower injection-related pain compared with the citrate-containing adalimumab reference product in both the Phase III PsO and RA trials.30
As clinical data in IBD have not been included in the regulatory submissions of adalimumab biosimilars approved to date, the use of these agents in these indications currently relies on extrapolation. Extrapolation of clinical efficacy and safety data to other indications of the reference product, not studied in clinical trials for the biosimilar, is fundamental to the concept of biosimilars. Extrapolation is possible and should be considered in light of the totality of evidence for a biosimilar (the analytical, functional [including mechanism of action], and the clinical and non-clinical data) along with adequate scientific justification. Additional data may be required to support extrapolation when it is not clear whether the efficacy and safety reported in one indication are relevant for another indication.20,31
Prof Reinisch summarised his presentation by explaining that clinicians need to be aware of the many considerations when selecting a biosimilar in order to reach a fully informed decision.
Alleviating Patient Concerns About Biosimilars: Challenges and Opportunities
Professor Alessandro Armuzzi
A comparison of surveys among IBD specialists performed in 2013 and 2015 highlighted that clinicians have become better informed about biosimilars and more confident in their use in clinical practice.32 However, this increased confidence is not always mirrored in patients. As a consequence, Prof Armuzzi explained the importance of ensuring that patients are well informed to maximise both the acceptance and clinical benefits of these agents.
The results of a survey conducted by the European Federation of Crohn’s and Ulcerative Colitis Associations (EFCCA),33 which evaluated patient (N=1,181) perceptions of biosimilars, were discussed during the symposium. Some patients were found to have concerns about biosimilars, particularly around efficacy, safety, and extrapolation to IBD, and wanted to know what biologic they were receiving (i.e., biosimilar or reference product). Overall, these findings highlight that patients with IBD require more information about biosimilars, and that they should be fully informed and involved in the treatment decision-making process. Increasing confidence in biosimilars and empowering patients can be facilitated by educating patients about the data supporting equivalence of a biosimilar to the reference product. For example, the totality of evidence concept should be explained, emphasising that the mechanism of action is the same between a biosimilar and reference product and that the extent of both analytical and functional data goes beyond a single clinical trial. Explaining these concepts to patients in a simple manner may help to improve their understanding of and confidence in biosimilars.
A considerable body of data from many fields of research has linked negative patient expectations to the occurrence of adverse symptoms and/or lack of efficacy, in turn impacting wellbeing of patients and treatment adherence. Such data suggest that a nocebo effect associated with a therapeutic intervention may be possible; the nocebo effect has subsequently been defined as an effect that is unrelated to the physiological action of the treatment and arises as a result of the psychosocial context or therapeutic environment on the patient’s mind, body, and brain.34 The potential for the nocebo effect has been reported in association with biosimilars.35 The underlying causes of the nocebo effect are complex and incompletely understood; some risk factors, such as clinical characteristics and symptom expectations, are pre-existing in the patient, whereas others can be acquired (e.g., through verbal suggestions).35 The importance of expectation in the context of the nocebo effect was discussed using the example of the opioid analgesic remifentanil. Expectation of a positive treatment outcome doubled the analgesic effect of the drug, whereas expectation of a negative outcome eliminated the analgesic effect.36
Given the potential risk of the nocebo effect when switching patients to biosimilars, clinicians should be familiar with strategies to prevent and manage its occurrence. At the heart of this lies communication between clinicians and patients. The BIO-SWITCH study37 evaluated the efficacy and safety of switching from infliximab reference product to CT-P13 in patients (N=192) with RA, psoriatic arthritis, or ankylosing spondylitis over a 6-month follow-up period. In this study, all patients received a letter about the option to transition to CT-P13 and a subsequent telephone follow-up; treatment was administered in group intravenous sessions. During this study, 24% of patients discontinued CT-P13, mainly due to subjective complaints that could possibly be explained by the nocebo effect. The BIO-SPAN study38 of patients (n=625) who underwent a non-mandatory switch from reference product to biosimilar etanercept used an enhanced structured communication approach. This involved a letter to patients about switching with a telephone follow-up and treatment was administered in individual subcutaneous sessions. Furthermore, the reduced costs associated with biosimilars were highlighted to patients, and healthcare professionals received soft skills training on potential objection handling and approaches to avoid the nocebo effect.38,39 A similar 6-month treatment persistence rate was observed following the switch compared with etanercept-treated patients in an historical cohort (N=600).38 Thus, good communication and education could have an effect on minimising the occurrence of the nocebo effect in combination with other approaches, such as building a strong clinician–patient relationship and identifying patients at risk (Figure 1).35,40,41
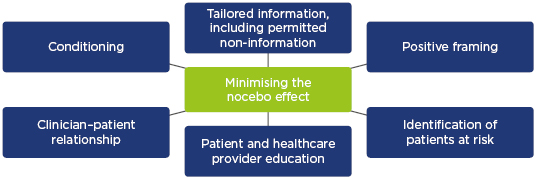
Figure 1: Strategies to minimise the occurrence of the nocebo effect.
Adapted from Armuzzi et al.40 and Kristensen et al.41
Prof Armuzzi concluded by explaining that several different communication strategies should be explored to prevent and manage the nocebo effect. These should include patient education and involve not just patients and clinicians but also other healthcare professionals, such as nurses, who play an important role in conveying information about biosimilars to patients.40
Concluding Remarks
Optimising the current management of IBD and developing strategies to predict and prevent IBD at the preclinical stage of disease are key elements in the evolving treatment landscape. Biosimilars of adalimumab are anticipated to play an important role in improving current care by providing cost savings and facilitating access to treatment. Clinicians should carefully consider the multitude of factors when selecting biosimilars, including the available totality of evidence, and should ensure that patients are informed, engaged, and empowered about their treatment through the use of effective communication strategies.
To view the webcast of the symposium, please click on the link below:
- Enter the password ‘UEGWVienna’ when prompted
- https://vimeo.com/302810410








