Speakers: Filip Baert,1 Vipul Jairath,2 Peter Irving3
Disclosure: Dr Baert has received research grants from AbbVie, Chiesi, Ipsen, MSD, and Roche; and speaker’s and consultancy fees from AbbVie, Falk, Ferring, Janssen, Mundipharma, MSD, Pfizer, Takeda, and Vifor. Prof Jairath has received consulting fees from AbbVie, Alimentiv (formerly Robarts Clinical Trials), Arena Pharmaceuticals, Bristol Myers Squibb, Celltrion, Eli Lilly, Ferring, Fresenius Kabi, GlaxoSmithKline, Genetech, Gilead, Janssen, Merck, Mylan, Pendopharm, Pfizer, Roche, Sandoz, Takeda, and Topivert; and speaker’s fees from AbbVie, Ferring, Janssen, Pfizer, Shire, and Takeda. Dr Irving has received research support or honoraria for acting in an advisory capacity or speaking on behalf of AbbVie, Warner Chilcott, Ferring, Falk Pharma, Takeda, MSD, Johnson and Johnson, Pfizer, Janssen, Shire, Vifor Pharma, Pharmacosmos, Topivert, Genentech, Hospira, Samsung Bioepis, and VHsquared.
Acknowledgements: Medical writing assistance was provided by Nicola Humphry, Nottingham, UK.
Support: The webinar and publication of this article were funded by AbbVie Inc.
Citation: EMJ Gastroenterol. 2021;10[Suppl 1]:2-9.
Meeting Summary
This webinar opened with an introduction to therapeutic drug monitoring (TDM) in inflammatory bowel disease (IBD) by Dr Baert, as well as an explanation of how this method developed as an aid for gastroenterologists to optimise the use of anti-TNF monoclonal antibodies (mAb) in patients whilst considering potential immunogenicity. Prof Jairath described the clinical studies that first indicated low serum mAb drug concentrations were associated with poor treatment response, and the subsequent trials that attempted to elucidate whether TDM might improve clinical outcomes. Dr Irving introduced the concept of treat-to-target (T2T), whereby patients’ symptoms and biomarkers are closely monitored, and used to make dose adjustments. He described the results of a study that investigated whether adding TDM to T2T regimens might improve clinical outcomes and explained that TDM may be more effective in a subset of patients rather than all patients with IBD treated with mAb. Together, the speakers considered the current guidelines for TDM in IBD, what the future may hold for this method, and answered questions posed by the audience.
Therapeutic Drug Monitoring in Inflammatory Bowel Disease: Where It All Started
Doctor Filip Baert
Dr Baert emphasised that TDM had come a long way since gastroenterologists first observed that episodic use of infliximab (IFX), an anti-TNFα mAb, could trigger the production of anti-IFX antibodies that were associated with an increased risk of infusion reactions, a reduced duration of response to treatment, and a reduction in serum IFX concentration.1 Concomitant treatment with immunomodulators (IMM) was found to counter this immunogenic response, improving IFX concentrations and reducing anti-IFX antibody levels.1 Although mAb are no longer used episodically, with treatment continuing as maintenance therapy, some patients still experience an immunogenic response. This is particularly common in patients with long intervals between doses, or those who do not reach sufficient drug levels after each dose.
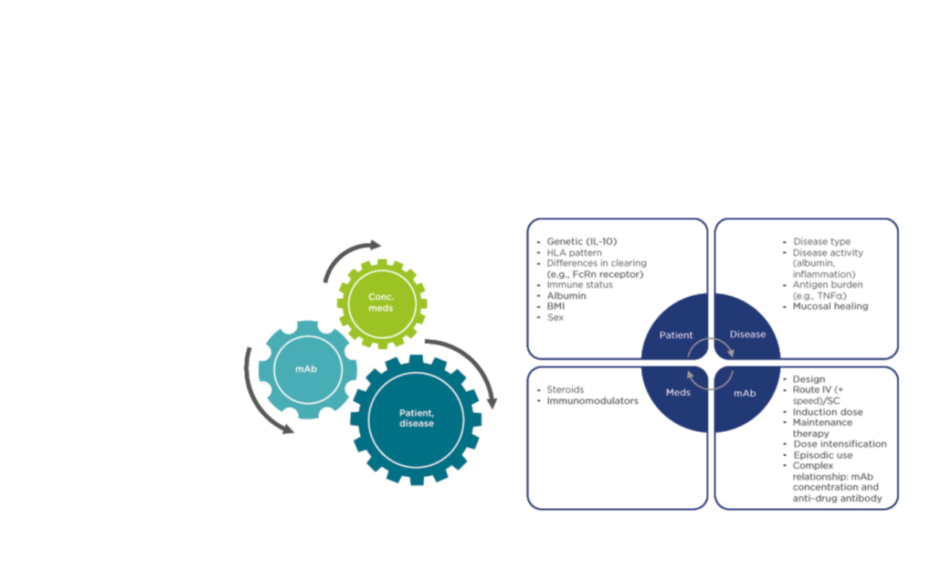
Because of the limited options originally available for IBD treatment, it was important that gastroenterologists optimised the dose of IFX or adalimumab (ADA), another anti-TNFα mAb, to provide each patient with the highest serum drug concentration possible. Dr Baert explained that the question now is whether clinicians can do this more rationally, guided by TDM. Anti-drug antibody monitoring may also be helpful, but it is important to note that the pharmacokinetics of mAb are completely different to those of small molecule inhibitors, and they can be affected by a wide variety of patient and disease characteristics, the use of concomitant medications, and the nature of the mAb itself (Figure 1).
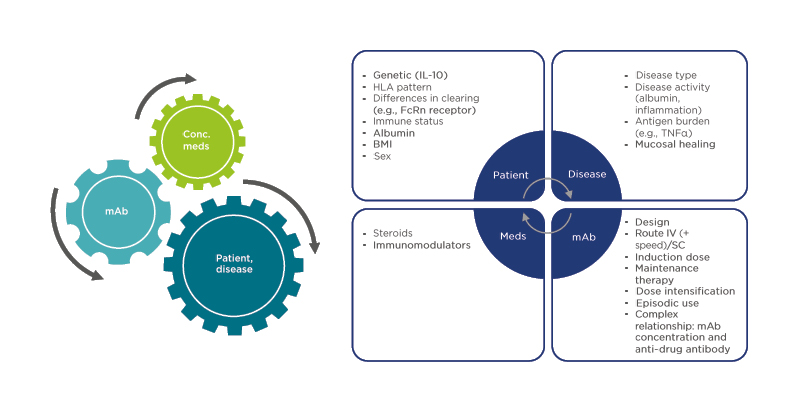
Figure 1: Pharmacokinetic confounding factors for monoclonal antibody therapy in inflammatory bowel disease.
Conc. meds: concomitant medicines; FcRn: neonatal Fc receptor; HLA: human leukocyte antigen; IV: intravenous; mAb: monoclonal antibody; Meds: medicines; SC: subcutaneous.
Early studies on the use of IMM therapy to improve duration of response to anti-TNFα mAb focussed on IFX as it tended to be associated with greater immunogenicity than ADA; however, IMM are also effective in combination with ADA,2 and may have benefits when combined with newer mAb such as vedolizumab (VLZ), an anti-α4β7-integrin antibody, in which the development of anti-drug antibodies has similarly been associated with reduced treatment efficacy.3
All mAb therapies have been associated with a clear dose–response effect; patients with lower serum concentrations have shown worse outcomes, and those with higher concentrations have showed better outcomes. For example, in a post hoc analysis of a randomised controlled trial of ADA and an IMM (azathioprine) in patients with Crohn’s disease (CD), higher ADA trough levels were associated with significantly better endoscopic response and mucosal healing at Weeks 26 and 52.4 Similar findings have been reported for VLZ and ustekinumab, an anti-IL-12/23 mAb.5,6
Dr Baert emphasised that although these findings do not necessarily indicate higher mAb concentrations will improve outcomes, many patients seem to be underdosed using current regimens. The difficulty of using TDM to optimise mAb therapy is that because of the various confounding factors involved, there is no absolute therapeutic range that we should target. Patients on maintenance therapy will usually do well on lower mAb concentrations but reaching harder endpoints, such as deep remission or fistula healing, requires higher concentrations. There is no evidence to suggest that higher concentrations of mAb are harmful; therefore, patients should be reassured that increasing dosage to reach higher mAb concentrations is unlikely to be associated with an increase in side effects.
Therapeutic Drug Monitoring in Inflammatory Bowel Disease: The Journey So Far
Professor Vipul Jairath
Prof Jairath explained that the personalised anti-TNF therapy in Crohn’s disease study (PANTS) was one of the largest prospective studies to monitor drug levels in patients with active CD.2 PANTS enrolled 955 patients starting treatment with IFX or ADA in routine clinical care. One of the key findings was that low drug concentrations at Week 14 were associated with both poor response at this timepoint, and with nonremission at Week 54. These results suggested that TDM could lead to improved outcomes, a possibility that has been investigated by several clinical trials.
One of the earliest trials was the TAXIT study in patients with CD or ulcerative colitis (UC) who were in stable remission on IFX maintenance therapy (N=263).7 TAXIT compared IFX dosing based on clinical symptoms plus C-reactive protein (CRP) levels (Group 1) versus dosing based on IFX trough levels (Group 2). Results showed that clinical remission at Week 52 was similar between the two groups, although more patients in Group 1 (17%) than in Group 2 (7%) needed rescue therapy during the maintenance phase (risk ratio: 2.4; 95% confidence interval: 1.2–5.1; p=0.018).
Several years later, the TAILORIX trial investigated dose modification in active luminal CD.8 Biologic-naïve adult patients (N=122) were treated with a combination of IFX and an IMM for 14 weeks, and then randomised to one of two TDM groups, or usual care. TDM Group 1 were uptitrated to IFX 7.5 mg/kg upon first clinical relapse (or fall in trough level), and to 10 mg/kg upon a second relapse. TDM Group 2 were uptitrated directly to IFX 10 mg/kg upon first clinical relapse, and the usual care group were uptitrated to IFX 10 mg/kg only upon elevated Crohn’s Disease Activity Index (CDAI). Results indicated that there was no significant difference between groups in terms of sustained steroid-free clinical remission from Week 22 to 54.
Considering the findings from the PANTS study, it is somewhat surprising that neither TAXIT nor TAILORIX showed a clear association between TDM approaches to dose modification and improved long-term remission. However, Prof Jairath pointed out that the TAXIT study population was already in stable remission, therefore drug doses in these patients may already have been maximally optimised. Additionally, the TAILORIX study population only received TDM-based dose modification after 14 weeks of therapy.7,8
Following an induction period, the SERENE-UC study randomised patients with moderate-to-severe UC (N=852) 2:2:1 to ADA 40 mg every week (qw), ADA 40 mg every 2 weeks (q2w), or exploratory ADA 40 mg with dose adjustments following a TDM regimen.9 Clinical remission at Week 52 in Week 8 responders was not significantly different between groups, though a numerically higher incidence of remission in the TDM and 40 mg qw groups was seen compared with the q2w group (37% and 40% versus 29%, respectively; Figure 2).
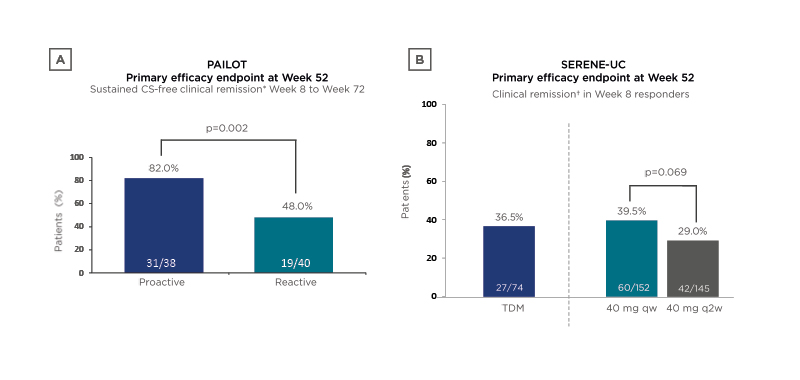
Figure 2: Results of two clinical trials investigating therapeutic drug monitoring in ulcerative colitis.
A) The SERENE-UC trial;9 B) The PAILOT trial.10
*Paediatric Crohn’s Disease Activity Index (PCDAI) <10.
†Full Mayo score ≤2 with no subscore >1.
CS: corticosteroid; qw: once per week; q2w: once every 2 weeks; TDM: therapeutic drug monitoring; UC: ulcerative colitis.
These findings suggested that in patients who were already responding to ADA therapy, there was no additional benefit in dose escalation based on TDM versus escalation to ADA 40 mg qw immediately following induction.
The PAILOT study investigated proactive versus reactive TDM-based treatment in biologic-naïve paediatric patients with CD who were responding to ADA after 4 weeks (N=78).10 In the proactive arm, serum ADA trough level concentrations drove dose modifications, whereas in the reactive arm, these modifications were only applied if there was a loss of clinical response. In this study, sustained corticosteroid-free clinical remission was achieved by significantly more children in the proactive TDM arm compared with the reactive-to-reactive TDM arm (82% versus 48%, respectively; p=0.002; Figure 2). It seemed possible, therefore, that TDM approaches had a significant effect on clinical remission in some IBD patient subgroups, but perhaps they may be less effective across a general population.
Prof Jairath summarised that, despite the predictive nature of serum mAb drug levels on long-term patient outcomes, the benefits of proactive TDM by itself remain unclear.
Therapeutic Drug Monitoring and Treat-to-Target: Perfect Partners?
Doctor Peter Irving
T2T is a therapeutic approach that has come into favour in IBD over recent years, with the aim of achieving disease remission by adjusting medication based on the attainment of predefined response targets. Dr Irving explained that the International Organization for the Study of Inflammatory Bowel Disease (IOIBD) initiated the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) programme in 2015 to determine the best goals for T2T in IBD. STRIDE recommended clinical and mucosal healing as suitable targets for IBD.11
One of the most profound studies to investigate the role of T2T in improving long-term outcomes in IBD was the CALM study.12 This trial enrolled patients with recent-onset CD who were naïve to biologics and immunosuppression (N=244). Following 8 weeks of steroid treatment, patients were treated with 160/80 mg ADA at Weeks 0 and 2 then 40 mg qw and randomised 1:1 at Week 0 to either clinical management (dose escalation from Week 12 driven by CDAI score and/or steroid use), or tight control (dose escalation from Week 12 driven by CDAI, steroid use, and/or biomarkers: faecal calprotectin [FCP] or CRP). At Week 48, mucosal healing (defined as CDEIS <4 with absence of deep ulcers) was seen in significantly more patients in the tight control group than the clinical management group (46% versus 30%, respectively; p=0.010),12 and these patients were less likely to experience disease progression over a median of 3 years.13 These findings suggested that dose escalations driven by biomarkers are more likely to result in mucosal healing, which is associated with improved long-term outcomes.
The SERENE-CD study investigated whether dose adjustments based on symptoms, biomarkers, and TDM (a TDM regimen) could improve outcomes when compared with adjustments based on symptoms and biomarkers alone (a clinically adjusted regimen).14 Following ADA induction therapy in patients with moderate-to-severe CD (N=184), responders were randomised to receive either a TDM-adjusted or clinically- adjusted maintenance regimen for 44 weeks. No significant difference was found between the two treatment regimens for key efficacy endpoints at Week 56, suggesting that adding TDM to a clinically driven dose adjustment regimen did not improve outcomes; however, compared with the biologic-naïve CALM population, patients enrolled in SERENE-CD had a longer disease duration, and many had already been exposed to IMM and/or biologics.
Despite the association between low mAb drug levels and poor response, clinical trials have so far not found that prospective TDM measurement improves clinical outcomes in patients with IBD. However, a recent post hoc analysis of the TAILORIX study may shed some light on this apparent contradiction.15 Following IFX dose escalation in 116 patients with CD, a significant decrease in FCP, but not CRP, was observed, and patients who achieved endoscopic remission at Week 54 had lower FCP levels 8 weeks after escalation. These findings suggested that TDM-driven dose escalation may improve clinical outcomes in patients with high FCP levels, but it may not add additional benefit to patients with lower FCP levels.
Dr Irving summarised that the evidence to date suggests that target mAb concentrations should be tailored for each patient, and that the need for TDM may be less important when clinical markers indicate adequate disease control in IBD.
Discussion
Doctor Filip Baert, Professor Vipul Jairath, and Doctor Peter Irving
Reactive Versus Proactive Treat-to-Target with Anti-TNF
Prof Jairath explained that he tended to use a reactive approach to TDM in his practice; if a patient presents with symptoms that suggest a disease flare, he assesses CRP and FCP levels, and if these indicate a loss of response then he will assess serum drug levels (Figure 3). Depending on the results, Prof Jairath would either optimise the dosage, or switch the drug class; however, Dr Baert was concerned that if clinicians wait until a patient is experiencing a disease flare, it may be too late to optimise their dosage to recapture a response. While he agreed that reactive TDM is a reasonable approach, he suggested that perhaps it could be combined with close monitoring of symptoms and biomarkers, so that dose adjustments can be made before a complete loss of response occurs. In response to Dr Irving’s concerns that performing regular blood tests after the induction period can be challenging, Dr Baert suggested that clinicians focus on patients at a higher risk of low mAb levels, such as those with high disease activity, low albumin, or those who have developed antibodies to a previous class of drug.
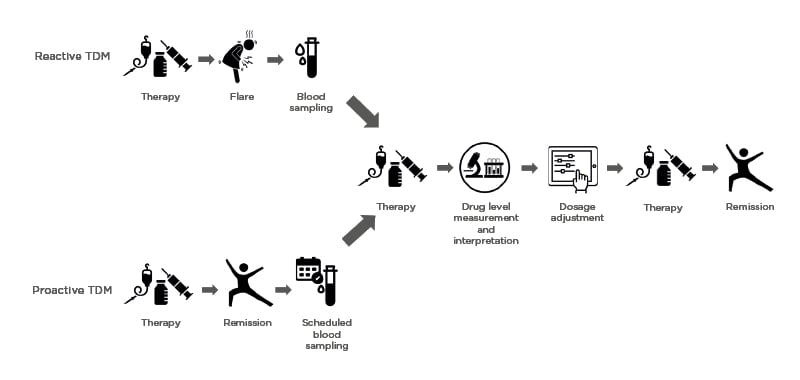
Figure 3: Differences between reactive and proactive therapeutic drug monitoring.
TDM: Therapeutic drug monitoring.
Prof Jairath suggested that dashboards could potentially be used to proactively determine drug dosage on a patient-by-patient basis.16
Dashboards use factors that have been associated with drug clearance, such as serum albumin, CRP, BMI, and sex, all of which are routinely tested in the clinic; however, Prof Jairath felt that more evidence was needed to indicate how this approach could be implemented in clinical practice.
Will Therapeutic Drug Monitoring Live and Die with Anti-TNF?
Anti-drug antibodies can be detected with a variety of different assays, some of which are drug sensitive, and some drug tolerant.17 Drug-sensitive assays are only able to detect free anti-drug antibodies, so when the drug itself is bound to these antibodies in immune complexes, the antibodies become undetectable.
Dr Irving explained that he currently uses a drug-sensitive assay for anti-drug antibodies, and therefore, more recently, only tests patients who have low serum drug concentrations.
If a patient with low drug levels is found to have high levels of anti-drug antibodies, then they may need to switch to an alternative drug. In clinics that use a drug-tolerant assay, it makes sense to test all patients, regardless of serum drug concentrations. Dr Irving feels it is safe to disregard transient and low-titre antibodies that may be reported by this type of assay, though their presence might prompt him to consider adding an IMM to a patient’s treatment, particularly in the context of borderline drug levels.
Dr Irving remarked that despite that evidence supporting TDM is not particularly robust, it is widely used. He felt that although international guidelines provided only weak recommendations for the use of TDM in IBD,18,19 there are times when TDM can provide information that is easy for a clinician to interpret and act upon. Dr Irving suggested that clinicians take a pragmatic approach to the use of TDM, at least until further data is available to direct its use.
With More Therapies Becoming Available with Different Pharmacokinetic/Pharmacodynamic Profiles, Will the Need for Therapeutic Drug Monitoring Change?
Dr Baert explained that the pharmacokinetic profiles of subcutaneous mAb formulations are quite different to intravenous formulations, making TDM less relevant for these drugs. He also emphasised that many of the new drugs being developed for IBD are humanised, or fully human, proteins, making immunogenicity less of an issue, which is likely to reduce the need for TDM in clinical practice in the long term. However, Dr Baert emphasised that small molecule therapies being developed to treat IBD will have completely new pharmacokinetic profiles, and that TDM may have a role to play in clinical trials to understand the effect of drug–drug interactions and renal function on serum concentrations. He also stressed that TDM should also continue to play a role in patients treated with the anti-TNF drugs that currently form the mainstay of IBD treatment.
Questions and Answers
Q: TDM Can Be Used for Population-Based Pharmacokinetic Modelling, but How Should It Be Leveraged for Individual Patients?
Prof Jairath emphasised that findings from TDM should always be considered alongside symptoms, disease biomarkers, and available imaging data before making any decisions on treatment. He suggested that dashboards might be one way to combine TDM with other measurements for personalised medicine purposes in the future. Dr Baert explained that he measures IFX drug levels at Week 6 but does not try to optimise dosage at this stage. Instead, he uses this information to differentiate between a primary nonresponder (with low drug levels immediately postinduction) and a patient experiencing loss of response during maintenance therapy (with adequate drug levels at Week 6 [IFX >12 mg/L], followed by low levels at a later timepoint).
Q: What Serum Drug Concentrations Levels Would You Target for the Newer Biologics?
Prof Jairath explained that we have far less data about the therapeutic doses of these drugs compared with anti-TNF therapy. Ustekinumab trough levels above 1 mg/L6 and vedolizumab levels above 30 mg/L5 appear to be associated with maintenance of clinical and endoscopic remission, respectively, but Prof Jairath felt that there were, as of yet, insufficient data to rely on these values for TDM.
Q: What are the Speakers’ Thoughts on the Data from the PANTS Study2 That Suggest Azathioprine May Have Efficacy in Crohn’s Disease Beyond Enhancing the Efficacy of Anti-TNF Therapy Through Reduction of Immunogenicity?
Prof Jairath explained that the PANTS study showed that adding an IMM to anti-TNF mAb therapy increased on-drug survival and time to immunogenicity compared with monotherapy through a mechanism that was independent of serum drug concentration. He felt that it is difficult to tell at this stage how much of the benefit from azathioprine was a result of improved mAb pharmacokinetics and direct IMM efficacy, and that both were probably involved. He would be interested to see whether azathioprine combined with one of the newer, less immunogenic IBD therapies would show any evidence of independent efficacy. Since IFX is a chimeric antibody and therefore more effective when combined with an IMM, Prof Jairath always confirms clinical and biochemical remission in a patient before considering IMM dose reduction, as well as checking that serum anti-TNF mAb levels are adequate.
Q: Studies Have Indicated That a Particular Genotype Is Associated with Increased Immunogenicity to Anti-TNF mAb. Will This Information Be Useful to Clinicians?
Prof Jairath explained that if a patient has developed high levels of anti-drug antibodies, the standard approach is to switch to a second anti-TNF drug. However, it has been suggested that patients are equally likely to develop antibodies to a second anti-TNF drug as they are to a first,20 and a recent study identified a genetic marker associated with a predisposition to developing anti-drug antibodies to anti-TNF agents.21 Although genetic testing is not routinely available at many institutions, Prof Jairath agreed that it would be useful to know whether a patient is at high risk of immunogenicity before starting anti-TNF mAb treatment.