Meeting Summary
In the first presentation, Prof Panaccione considered how early treatment of Crohn’s disease (CD) is key for achieving the therapeutic goals, which include symptomatic remission and mucosal healing. The latest STRIDE guidelines,1 published in 2015, endorse endoscopic remission defined as “resolution of ulceration at ileocolonoscopy”, and emphasised the need for tight monitoring of inflammation. He explored data highlighting how the ability to achieve mucosal healing decreases with increased disease duration, that benefits from mucosal healing may not be realised until the second year of treatment, and how patients who experience mucosal healing are less likely to be hospitalised and require surgery. Studies show patients do better with the ‘top-down’ approach, receiving anti-tumour necrosis factor (TNF) drugs early in the disease course, which has led to the introduction of a treatment algorithm suggesting patients with high-risk factors for poor prognosis should receive early ‘top-down’ therapy and lower-risk patients traditional ‘step-up’ therapy. The need for decisive early treatment to slow progression emphasises the importance of facilitating early diagnosis, and identifying patients for early biologic therapy. In the second presentation, Dr Iris Dotan explored data suggesting that optimal positioning for vedolizumab appears to be early in the course of disease. Furthermore, vedolizumab’s effect on clinical remission improves over time, clinical remissions have been shown to be maintained long-term, and vedolizumab reduces rates of hospitalisation. A favourable risk-benefit profile for vedolizumab has been shown for long-term use with no increase in the incidence of adverse events in the 5-year analysis. There are now 77,382 patient-years of post-marketing exposure to vedolizumab worldwide.2 The latest European Crohn’s and Colitis Organisation (ECCO) guidelines recommend the use of vedolizumab in patients with moderate to severe localised ileocaecal and colonic CD refractory to steroids and/or anti-TNF-αs.
Slowing Progression of Crohn’s Disease: Decisive Treatment for Early and Long-Term Remission
Professor Remo Panaccione
Since 1995 there has been an evolution in the management of CD, starting first with 5-aminosalacylic acid (5-ASA), steroids, and azathioprine,3,4 then moving around the year 2000 to anti-TNF-α biologics,5-7 in 2013 to biosimilars8,9 and vedolizumab,10 and finally in 2016 to ustekinumab.11 Throughout this time surgery has remained an option in CD. With so many possible treatments, clinicians need to consider the ‘patient journey’ and identify patients early enough to receive the right drug at the right time.
The biologic revolution began almost 20 years ago with the approval of infliximab for moderate-to-severe CD.6 Despite the subsequent development and approval of newer anti-TNF-αs, overall induction of remission in randomised controlled trials has remained between 30% and 50%5,12-14 and maintenance of remission between 20% and 40%.14-17 Such data, explained Prof Panaccione, demonstrates that therapeutic gaps still exist in treatment of inflammatory bowel disease (IBD), underlining the need for new agents.
Many important lessons have been learnt from anti- TNF-αs. These agents are generally safe (although some issues remain); antibodies are detrimental for efficacy, adequate drug levels are good, and data suggest the benefit of combination therapy (anti-TNF-α plus immunomodulators). Furthermore, paradigms have changed in the anti-TNF-α era with the recognition that treating early is beneficial, and treating beyond symptoms decreases rates of surgery.1
CD is a chronic progressive disease inducing cumulative structural damage. A 2011 study by Pariente et al.18 (using Lémann scores to measure cumulative structural damage) showed that with time CD patients are more likely to develop strictures, fistulae/abscesses, and undergo surgery (Figure 1). The goal for CD is to treat early and control inflammation.
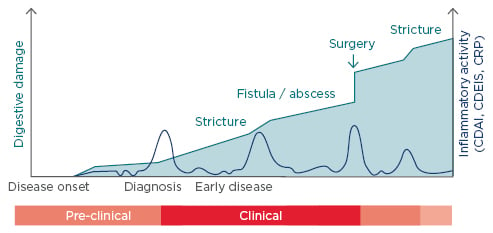
Figure 1: The progression of digestive damage and inflammatory activity in a theoretical patient with Crohn’s disease.18
CDAI: Crohn’s Disease Activity Index; CDEIS: Crohn’s Disease Endoscopic Index of Severity; CRP: C-reactive protein.
Desired outcomes differ between early and late- stage CD. For early disease, they include complete absence of symptoms, no disease progression, no complications or disability, and normal quality of life (QoL), while for late-stage disease they include stabilisation of non-inflammatory symptoms, no progression of damage or disability, and improved QoL. The difference in goals emphasises that if the early treatment window of opportunity is missed, symptomatic remission will not be achieved. This is because patients may be left with chronic abdominal pain, or functional consequences leading to chronic diarrhoea, short bowel syndrome, and/or loss of the ileocaecal valve.
The concept of early treatment of disease needs to be reinforced, said Prof Panaccione, because it is here that the greatest benefits can be derived from therapy. New data presented at ECCO 2017 suggests that the window of opportunity may in reality be months rather than years. A retrospective analysis of the Alberta IBD Consortium, undertaken by Prof Panaccione and colleagues, showed CD patients prescribed thiopurines or anti-TNF-α agents at the inflammatory stage were significantly more likely to avoid surgery compared with those initiating treatment after complications (penetrating, ileal stricturing, or stricturing) had developed.19
Differences in early disease biology were highlighted by a subgroup analysis of the EXTEND study showing rates of healing with adalimumab decrease with disease duration. The data showed that patients with <2 years disease had mucosal healing rates of 44%, patients with 2–4 years of disease had mucosal healing rates of 40%, and patients with ≥5 years had mucosal healing rates of 21%.20 Such data, said Prof Panaccione, emphasises how early intervention can result in better outcomes.
The REACT trial, randomising community gastroenterology practices to conventional management or early combined immunosuppressants (anti-TNF-αs and antimetabolite), showed that the benefits of early treatment may not be realised until the second year of therapy.21 The study, exploring whether patients achieved remission (being off steroids) at different time points, showed combined immunosuppression did not deliver symptomatic remission benefits until after Month 24 of treatment (p=0.959 at Month 6, p=0.517 at Month 12, p=0.441 at Month 18, and p=0.083 at Month 24). At 24 months, the composite of major adverse outcomes (occurrence of surgery, hospital admission, or serious disease-related complications) was 27.7% for combined immunosuppression versus 35.1% for conventional treatment (hazard ratio [HR]: 0.73, p<0.0003).
An endoscopy sub-study of the ACCENT I trial (the infliximab trial) demonstrated that success of mucosal healing can be related directly to rates of hospitalisation.22 Among patients who achieved mucosal healing at both Week 10 and Week 54 after treatment, none required hospitalisation, compared to 18.8% of patients with mucosal healing at only one of those visits and 28% with no mucosal healing at either visit.22
The STRIDE treat-to-target recommendations, written in 2015 by 28 IBD specialists, define composite endpoints of clinical/patient-reported outcome remission (defined as resolution of abdominal pain and normalisation of bowel habit) and endoscopic remission (defined as resolution of ulceration at ileocolonoscopy).1 According to STRIDE, biomarkers (C-reactive protein and calprotectin) can be considered as adjunctive measures of inflammation, but not targets for CD monitoring. While histologic remission is not currently considered a target, said Prof Panaccione, new data is anticipated later this year exploring whether surrogate markers could be used for treat-to-target beyond improvements in symptoms.
Results of the Top-Down versus Step-Up Trial, comparing early use of combined immunosuppression (infliximab and azathioprine; ‘top-down’) with conventional management (corticosteroids and infliximab when needed; ‘step-up’), suggested initiating more intensive treatment early in disease leads to better outcomes.23 At the end of 2 years, 73% of patients who received anti-TNF-αs as their initial therapy showed disappearance of ulcers compared with 30% receiving traditional step-up therapy (p<0.001).23
A subset analysis of 49 patients (taken from a study by Baert et al.24 of 133 patients comparing the combination azathioprine and infliximab with conventional steroids) showed mucosal healing (defined as an endoscopic score of 0) after 2 years treatment predicts long-term benefits. At Year 3+4, remission occurred in 71% of patients with mucosal healing after 2 years versus 41% without (p=0.073), remission off steroids occurred in 71% with mucosal healing after 2 years versus 27% without (p=0.05), and remission off steroids and no anti-TNF-α occurred in 63% with mucosal healing at 2 years and 18% without (p<0.05). Further insights into mucosal healing are likely to come from the ongoing REACT II study (currently two-thirds enrolled), which provides escalated therapy at Week 16, 32, and 48 if patients have not shown ileocolonoscopy improvements.
The holy grail for treat-to-target, said Prof Panaccione, is to demonstrate that mucosal healing changes long-term patient outcomes. Despite the wealth of evidence for biologic therapies (mostly anti-TNF-αs), uptakes around the world remain low. A 2015 review of the epidemiology of CD, by Burisch and Munkholm, showed that earlier and more frequent use of immunomodulators/biologics in CD to be associated with reduced surgery.25 The greatest fears for patients with CD are short and long-term side effects of therapies, with most wanting to come off therapy when they are well. Such concerns emphasise the importance of developing treatments that are not only safe and effective upfront, but also have long-term safety profiles.
Taking account of the possibility that long-term use of anti-TNF-αs prevents bowel damage, a 2011 treatment algorithm suggested patients with high risk factors for poor prognosis (including diagnosis at age <40 years, extensive anatomic involvement, perianal or severe rectal disease, deep ulcers, prior surgical resection, and structuring and/or penetrating behaviour) should receive early ‘top-down’ intervention with biologic therapy.26 For lower-risk patients, traditional ‘step-up’ therapy can be used with biologic therapy introduced if they fail to respond to therapy.
A financial analogy, suggested Prof Panaccione, can be used to explain the benefits of early treatment in CD. Just as people investing early and progressively during their working lives are wealthier in retirement than those waiting until later to invest, people with CD who have anti-TNF-α treatments early do better in the long-term.
The need for decisive early treatment to slow progression emphasises the importance of facilitating early diagnosis and identifying appropriate patients for early biologic therapy. The STRIDE recommendations should be adopted for a treat-to-target approach,1 with a rapid step-up approach being the rule, and tight monitoring of objective signs of inflammation (mucosal healing). The ultimate therapeutic goal for CD, concluded Prof Panaccione, is to return patients to normal life.
Early Use of Gut Selective Therapy in Crohn’s Disease for Long-Term Remission
Doctor Iris Dotan
In the treatment of CD and ulcerative colitis (UC), there is currently a move away from the old goals of improving symptoms,1,27 achieving a clinical remission,1 or even a steroid-free clinical remission,28 to new ambitions around mucosal healing1,24,27 that ultimately achieve the goal of changing the course of disease.1,27
The evolving IBD environment makes it important to consider whether conventional therapies (5-ASA, steroids, thiopurines, methotrexate, and anti-TNF-α) meet the new treatment goals. Studies suggest that thiopurines and anti-TNF-α agents meet short-term end points of clinical remission, steroid-free clinical remission, and clinical and mucosal remission. It is less certain however, whether long-term goals of disease modification (involving reduction of surgical risk, disability, and damage) are significantly affected. For thiopurines there is conflicting evidence regarding reductions of surgical risk,29 with only anti-TNF-α shown to decrease the risk of surgery in CD and UC.30
Studies show the ‘top-down’ approach, using effective therapy early in disease, delivers benefits including higher efficacy, lower disease-related complications, higher mucosal healing and decreased rates of surgery and hospitalisation.27 However, the downsides of these treatments include higher risks of drug-related serious infections and increased costs.27 Such consideration led the 2017 ECCO consensus on diagnosis and medical management of CD to conclude there is a “complex benefit-risk balance” for early aggressive therapeutic strategies using immunosuppressants and biologics in CD.31
Clinicians need to be aware that the window of opportunity is small and that in IBD early intervention is key to prevent disease progression. A longitudinal prospective observational follow-up study in 154 newly diagnosed CD patients, by Dr Dotan and colleagues, showed that by 10 months 40% had experienced disease complications (defined as progression to complicated disease, CD-related hospitalisations, or surgeries).32 Such data, said Dr Dotan, suggest there is a need to intervene quite fast.
Vedolizumab, a gut selective anti-trafficking agent, is a newer option for the treatment of CD. Vedolizumab works by blocking α4β7 integrin (found on the surface of leukocytes), preventing them from binding to mucosal addressin cell adhesion molecule-1 (MAdCAM-1) ligands (found on gut endothelial cells).33,34
Vedolizumab has been shown to improve outcomes in anti-TNF-α naïve patients. A post hoc analysis of the GEMINI II and GEMINI III trials showed rates of response and remission were higher in patients receiving vedolizumab as a first-line biologic than for patients who experienced prior anti-TNF-α failure (Figure 2).35 The analysis, involving 516 anti- TNF-α naïve patients and 960 anti-TNF-α failure patients, showed for both groups that beneficial effects of vedolizumab increased with time.
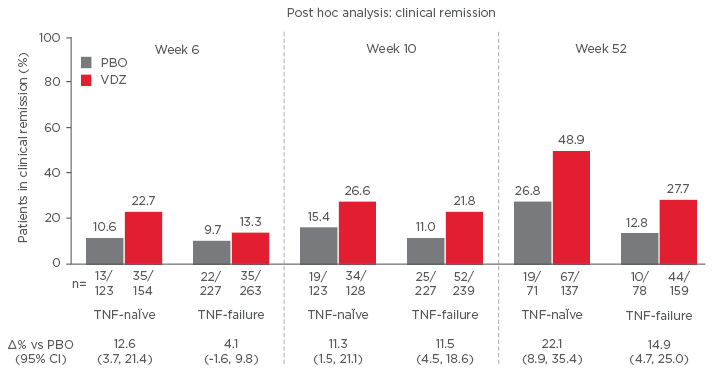
Figure 2: Biologic-naïve Patients have more prominent clinical remission (GEMINI II and III Pooled).45
CI: confidence interval; PBO: placebo; VDZ: vedolizumab; TNF: tumour necrosis factor.
A systematic meta-analysis presented at ECCO 2017 (98 studies involving 1,010 CD and 704 UC patients) further supports the concept of improvement associated with vedolizumab over time.36 Results show pooled clinical remission was 24% at Week 6, 30% at Week 14, 23% at 6 months, and 30% at 1 year, and that pooled steroid-free clinical remission was 13% at Week 6, 25% at Week 14,23% at 6 months, and 25% at 12 months.36
Undoubtedly, one of the most important new goals for CD treatments is to produce mucosal healing. A promising signal for vedolizumab comes from a 2017 retrospective chart review of 32 colonoscopies undertaken in 23 CD patients enrolled in the GEMINI long-term safety study. Results in patients (who had at least 1 year of continued vedolizumab treatment) showed 44% of colonoscopies were associated with complete healing, 47% with partial healing, and 9% with no healing.37
Additionally, three different ‘real-world’ cohort studies suggest benefits for vedolizumab in achieving mucosal healing. The first study (University of Chicago, Chicago, Illinois, USA) showed mucosal healing occurred in 17% after 3 infusions;38 the second (Mayo Clinic, Rochester, Minnesota, USA) showed mucosal healing occurred in 20%;39 and the third study (Washington University, Seattle, Washington, USA) showed at Week 14 mucosal healing occurred in 30% and endoscopic improvement occurred in 52% (Figure 3).40 Finally, in the VICTORY consortium study, which included 212 patients with moderate-severe CD, mucosal healing occurred in 20% of patients at Week 26, rising to 63% of patients at Week 52.41
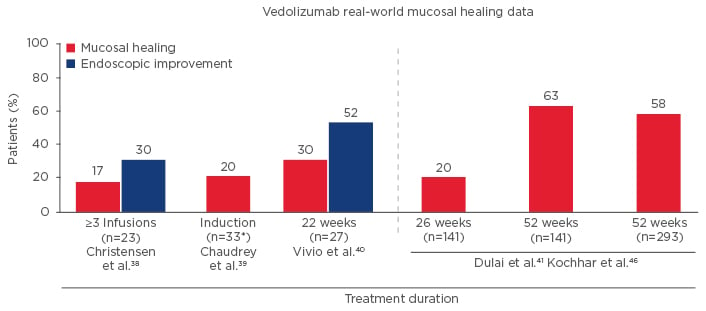
Figure 3: Mucosal Healing with Vedolizumab for Treatment of Crohn’s Disease in the Real World.40
What will be of vital importance, said Dr Dotan, is the ability to determine predictors of remission and mucosal healing since this helps therapies to be positioned in the clinical setting. From the US VICTORY Consortium data, it is clear patients with no prior anti-TNF-α exposure experienced more clinical remission than patients with anti-TNF-α exposure (HR: 0.40) and that patients with moderate disease experienced more clinical remission than those with severe disease (HR: 0.54).41 Regarding mucosal healing, patients with no anti-TNF-α exposure experienced more mucosal healing than patients with anti-TNF-α exposure (HR: 0.29), and patients with moderate disease experienced more clinical remissions than those with severe disease (HR: 0.54).41
New data presented at ECCO 2017 reported on the effectiveness and safety of vedolizumab in CD patients who had completed GEMINI II and enrolled in the GEMINI open label extension study. Data showed long-term vedolizumab therapy (˜5-years) was associated with clinical benefits including clinical response, clinical remission, and health-related QoL improvements in patients with moderate-to-severe active CD.42
Another study, comparing vedolizumab (n=81) versus infliximab (n=162) in biologic-naïve IBD-patients suggested that at 12 months vedolizumab was associated with a reduction (12.3%) in hospitalisation compared to infliximab (17.9%); although the result was not statistically significant.43
Confidence in the safety of vedolizumab was reported by Colombel et al.44 showing no increase in the incidence of adverse events (including gastrointestinal adverse events, infections and serious infections) with long-term exposure of over 3 years. Notably, as of November 2016, vedolizumab has 77,382 patient-years of post-marketing exposure worldwide.2
The latest ECCO guidelines state: “decisive treatment with a potent agent (‘top-down’ approach) at an early stage may be preferred by the patient suffering symptoms from active disease”. The guideline continues: “The therapeutic goal should be to induce clinical remission for every patient, but even at diagnosis it is essential to keep in mind how remission will be maintained after medical induction therapy”.31
Dr Dotan concluded that vedolizumab is associated with improved remission in anti-TNF-α naïve patients, suggesting optimal positioning early in the disease course. The favourable risk-benefit profile of vedolizumab shown in the 5-year analysis supports its early and long-term use in moderate- to-severe CD. ECCO guidelines recommend the use of vedolizumab in patients with moderate-to-severe localised ileocaecal and colonic CD refractory to steroids and/or anti-TNF-αs.








