Meeting Summary
Frank Tacke presented the hypothetical case of a 24-year-old female with a 10-year history of multiple emergency department (ED) visits for recurrent severe abdominal pain with weakness, fatigue, mental fogginess, and dark urine. Laboratory tests, imaging, and endoscopies were generally unremarkable, but urine analysis showed elevated porphobilinogen (PBG), 5-aminolevulinic acid (ALA), and uroporphyrin, leading to a diagnosis of acute hepatic porphyria (AHP).
Awareness of this rare disease is low, with misdiagnosis/delayed diagnosis common, therefore AHP should be considered in patients with the above-mentioned symptoms. David Cassiman outlined current management strategies for AHP, including the avoidance of attack triggers (e.g., fasting, smoking, alcohol, drugs); the treatment of acute attacks (e.g., haemin, glucose, analgesics); and the management of chronic symptoms. He also discussed a new treatment, givosiran▼ (Givlaari®▼, [Alnylam Pharmaceuticals Inc., Cambridge, Massachusetts, USA]). In the ENVISION study, 94 patients with AHP were randomised to givosiran▼ or placebo for 6 months, followed by a 30-month open-label phase. At a 24-month interim analysis, the median annualised attack rate (AAR) had fallen from 1.04 (double-blind givosiran▼) to 0.00 (open-label givosiran▼), and from 10.65 (double-blind placebo) to 1.35 (open-label givosiran▼). Secondary efficacy and quality of life results supported the sustained benefits of givosiran▼, which had an acceptable safety profile. The question and answer session elicited information about the diagnostic management of patients with unexplained abdominal pain episodes; which patients are most likely to benefit from givosiran▼; the reasons for misdiagnosis/delayed diagnosis, AHP symptoms and management, which patients can receive givosiran▼; the accuracy of PBG testing; and the importance of renal and liver function monitoring for patients on givosiran▼.
Mystery Diagnosis, Disease Overview, and Pathophysiology
Frank Tacke
Details of a hypothetical 24-year-old female with a longstanding history of recurrent abdominal pain were presented. Over the previous 10 years, she had presented at the ED once or twice yearly with episodes of severe abdominal pain. At her last ED visit, her abdominal pain was debilitating and overwhelming (9–10/10 in severity). Liver function tests were mildly elevated, but blood counts, inflammatory markers, renal function tests, and urine analysis were all normal. Abdominal imaging (ultrasound and CT) and gynaecological examination were also normal. She was discharged from the ED without a specific diagnosis, but was referred for oesophagogastroduodenoscopy and colonoscopy, which were normal.
Her severe abdominal pain began 10 years ago with the onset of menses. She recalls symptoms of weakness, fatigue, and mental fogginess preceding episodes of abdominal pain. The pain was usually localised to her lower abdomen, was crampy/colicky in nature, and 8–10/10 in severity, which prompted seeking medical attention. There were no identifiable precipitants, although it seemed to occur around the time of menses. Her pain usually required an ED visit, but generally improved over 3–5 days after receiving intravenous fluids and opiates. The episodes were associated with feeling dehydrated and producing darker (reddish) urine. She estimated eight discrete attacks from age 18 to 22 years but had months between attacks in which she was completely asymptomatic.
The hypothetical patient was also affected by anxiety, had a maternal history of hypothyroidism, and had her appendix removed 4 years ago for suspected appendicitis. She had no known drug allergies, and was taking fluoxetine, oral contraceptives, oxycontin, and analgesics as required. She was a radiology technician who does not smoke or use drugs and only drinks 1–2 glasses of wine 2–3 times per month.
Her most recent episode led to an ED visit for abdominal pain 3 weeks ago. She had severe abdominal pain (9/10) with nausea and fatigue and noted dark urine prior to the ED visit. She had radiating limb pain (mainly in her legs). Her heart rate was 110 bpm and her blood pressure was 154/92 mmHg. Physical examination showed severe diffuse abdominal pain that was non-localised. Laboratory tests revealed mild hyponatraemia (129 mEq/L) and elevated liver function tests (alanine aminotransferase: 65 U/L; aspartate aminotransferase: 50 U/L). Other tests (e.g., urine pregnancy, complete blood count, urine analysis, C-reactive protein, erythrocyte sedimentation rate) were normal.
Abdominal and pelvic CT with contrast were unremarkable, as was an ultrasound of her right upper quadrant. Her creatinine kinase was normal (120 U/L) and a hepatitis panel was negative. Analysis of her urine showed elevated PBG (79 mg/g of creatinine [reference range:1 0–4 mg/g]), ALA (35 mg/g [reference range:1 0–7 μg/g]) and uroporphyrin (98 μg/g of creatinine [reference range:2 0–30 μg/g]), resulting in a diagnosis of AHP.2-4
It is very important that porphyria is considered as a differential diagnosis in patients with recurrent abdominal pain with neurological symptoms, muscle weakness, or fatigue. The objectives of this webinar were to:
raise awareness of AHP and highlight the importance of achieving an earlier diagnosis;
discuss best practice for the diagnosis and management of AHP; and
present the latest clinical data supporting givosiran▼ (Givlaari▼), which is indicated for the treatment of AHP in adults and adolescents aged ≥12 years.5
Patients with acute porphyrias have altered haem biosynthesis, which results in toxic metabolites.6 As AHP is a rare disease, it is often not considered in differential diagnosis when assessing for unexplained acute abdominal pain (and other characteristic symptoms).4 AHP has a variable presentation, with many of the symptoms presenting as non-specific and mimicking other more prevalent conditions, leading to challenging disease identification and delayed diagnosis or misdiagnosis.3,7,8
Current management strategies for AHP focus on the avoidance of attack triggers (such as fasting, alcohol, smoking, and certain medications), the treatment of acute attacks (with fluids, glucose infusions, pain medications, and haemin), and the management of pain and other chronic symptoms.1,3 However, medications may have unanticipated deleterious effects.1
The toxic metabolites produced in AHP do not only affect the liver, but can also cause a myriad of other problems, including central nervous system (CNS),9-11 peripheral nervous system (PNS),9,10 and autonomic nervous system (ANS) manifestations4,9 (Figure 1). Patients with variegate porphyria and hereditary coproporphyria can also have cutaneous manifestations (lesions on sun-exposed skin).11 Furthermore, AHP can result in long-term complications, including hepatocellular carcinoma, chronic kidney disease (CKD), neuropathy, and hypertension.9,12
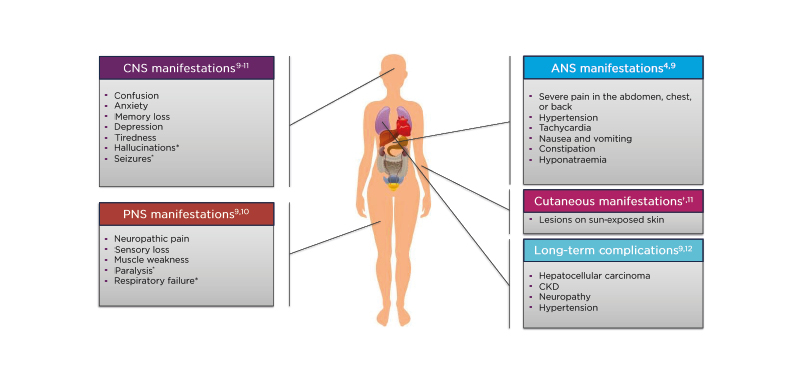
Figure 1: Clinical characteristics of acute hepatic porphyria and associated conditions.
*Only occurs in severe cases.
†Only occurs in variegate porphyria and hereditary coproporphyria.
ANS: autonomic nervous system; CKD: chronic kidney disease; CNS: central nervous system; PNS: peripheral nervous system.
Patients with AHP can also experience chronic symptoms between attacks. In the prospective, multinational EXPLORE study,13 65% of patients with AHP and recurrent attacks experienced chronic symptoms, with 46% of patients experiencing them daily. The most common symptoms were abdominal pain (20%); tiredness, anxiety, and nausea (each 19%); headache, weakness, and trouble sleeping (each 14%); and back pain (12%).13
Patients with AHP are frequently misdiagnosed (e.g., non-specific abdominal pain, irritable bowel syndrome, depression, or fibromyalgia)14 and can even undergo potentially unnecessary surgeries.4 Frequent healthcare utilisation, reduced quality of life, and lost workdays all contribute to disease burden.13,15,16 Further, the pain and the unpredictable nature of attacks (Figure 2) is a source of fear and anxiety for many patients.
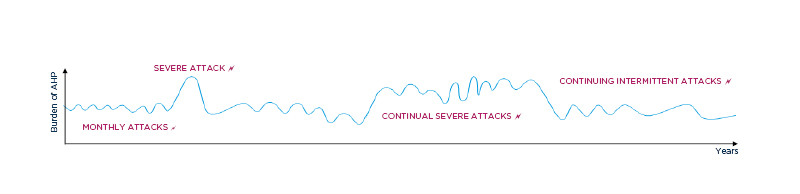
Figure 2: Illustrative patient experience with acute hepatic porphyria.
AHP: acute hepatic porphyria.
Potential long-term complications of AHP include liver and kidney disease, hypertension, and chronic neuropathy.1,17-22 AHP has been identified as a risk factor for primary liver cancer, especially hepatocellular carcinoma.17 In a Norwegian cohort study, the annual incidence of primary liver cancer was 0.35% in individuals with AHP, over 100 times higher than the 0.003% in a reference population.18 Porphyria has also been linked to CKD.19 In a French study, 59% of patients with symptomatic acute intermittent porphyria (AIP) had CKD.20 In a Spanish study, patients with sporadic AIP (<4 attacks/year) had a significantly higher risk of CKD than patients with latent AIP (30% versus 0%; p=0.018).21 Patients with AHP may also be at increased risk of chronic sustained hypertension,1,22 although as the risk of hypertension is high in the general population, further research is required to ascertain the true excess risk associated with AHP.22 Patients with AHP can also develop chronic pain associated with axonal motor polyneuropathy.1 Chronic pain symptoms can lead to severe depression and anxiety, which may necessitate psychiatric care,1 and suicidality has been observed in patients with AHP.23 Lastly, severe attacks can even result in permanent quadriplegia.12
Many different medical specialists may be variably involved in the diagnosis and management of patients with AHP.7 AHP commonly presents with acute neurovisceral attacks that manifest as severe abdominal pain and debilitating chronic symptoms.13 Prompt diagnosis and treatment is beneficial as this may improve prognosis and prevent severe or chronic neuropathic symptoms.4 Therefore, gastroenterologists play a vital role in identifying the hallmark symptoms of AHP in order to obtain an earlier diagnosis and improve the management of these patients.
AHP can be diagnosed with a random (spot) urine test for PBG, ALA, and porphyrins, normalised to creatinine.11,24 The ideal time to take a urine sample for diagnosis is during a suspected attack.25 Genetic and biochemical testing can then be used to confirm the diagnosis and ascertain the type of AHP.6,26 Sequencing and deletion testing detects approximately 95–99% of mutations in the genes associated with AHP.26 However, due to a lack of awareness, incorrect tests are often ordered when AHP is suspected.27 Testing for porphyrins alone cannot diagnose AHP, as levels can be elevated in various disorders.11
Disease Management and Givosiran▼ Treatment
David Cassiman
Current management strategies for AHP focus on the avoidance of attack triggers (e.g., smoking, alcohol, drugs, and hormonal therapy), the treatment of acute attacks, and the management of pain and other chronic symptoms.3
Acute attacks can be treated with haemin, which decreases ALAS1 activity and urine and plasma ALA and PBG.1,3 Glucose and carbohydrate loading can also be used to downregulate the haem biosynthesis pathway,1,3 and may be most effective in patients who are malnourished or where dietary restrictions have contributed to an attack.24 Pain is generally managed using opioid and non-opioid pain medications.1 For women who experience acute attacks related to their menstrual cycle, gonadotropin-releasing hormone agonists may be used to suppress ovulation.1 Patients with AHP may also benefit from treatment for their other symptoms, e.g., nausea, hypertensive crises, neuropathy, seizures, metabolic changes, anxiety, and depression. For severely affected patients, liver transplantation may be considered as a last resort.1
Givosiran▼ has recently become available as a treatment option for AHP.1,3 This small interfering RNA (siRNA) therapeutic reduces ALAS1 messenger RNA, thus lowering ALA and PBG accumulation, which have been linked to attack frequency and symptoms.5,28 Givosiran▼ is indicated for the treatment of AHP in adults and adolescents aged ≥12 years.5
In the ENVISION study, 94 patients (aged ≥12 years) with AHP (≥2 attacks in the previous 6 months) were enrolled at 36 sites in 18 countries.29 Patients were randomised to monthly subcutaneous givosiran▼ 2.5 mg/kg or placebo.29 The primary endpoint was the AAR (i.e., attacks that required hospitalisation, urgent healthcare, or at-home haemin administration per year) among 89 patients with AIP.29 Secondary endpoints included urinary ALA and PBG, haemin use, pain, fatigue, nausea, and quality of life among 89 patients with AIP; and AAR among all 94 patients with AHP.29
Among the 94 patients with AHP, the median age was 38 years, 89% were female, and the median time since diagnosis was 7 years.29,30 The median historical AAR was 8.0, 40% had prior haemin prophylaxis, 52% had symptoms most/every day between attacks, and 29% regularly used opioids.29,30 Baseline median urinary ALA and PBG were 16.4 and 39.6 mmol/mol creatinine, respectively.30
After the 6-month double-blind period, 93 eligible patients entered a 30-month open-label extension, during which patients received monthly givosiran▼ 2.5 or 1.25 mg/kg, although this was later increased to 2.5 mg/kg for all patients.30 At a 24-month interim analysis (at which time all patients had ≥18 months of follow-up), the median AAR had fallen from 1.04 during the double-blind period to 0.00 during the open-label period among patients who received givosiran▼ throughout, and from 10.65 to 1.35 (i.e., an 87% reduction) among those who received placebo followed by givosiran▼ (Figure 3).30
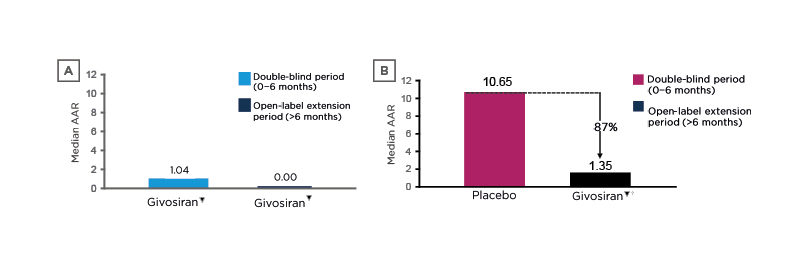
Figure 3: Reductions in annualised attack rate* with givosiran among A) patients who received givosiran during the double-blind and open-label periods and B) those who received placebo during the double-blind period and givosiran during the open-label period (descriptive analyses).30
*Attacks that required hospitalisation, urgent healthcare, or at-home haemin administration per year.
†Placebo crossover patients receiving givosiran 2.5 mg/kg (n=29) or 1.25 mg/kg (n=17).
AAR: annualised attack rate.
The proportions of patients free from attacks during each 3-month interval increased among those randomised to givosiran▼, from 0% (baseline) to 67% (Months 3–6, i.e., the second half of the double-blind period) to 83% (Months 21–24) to 95% (Months 27–30) and among those originally randomised to placebo, from 2% to 24% to 76% to 94%, respectively.30 Patients who were randomised to givosiran▼ had median annualised days of haemin use of 0.0 during the double-blind and open-label periods, while those randomised to placebo had 15.0 annualised days of haemin use during the double-blind phase, which fell to 0.7 during the open-label phase, a reduction of 95%.30
Givosiran▼ was also associated with clinically important (based on data from patients with other chronic diseases31-34) improvements in quality of life.30 Mean increases in the Short-Form 12 physical component summary score were 5.1 during the double-blind period and 8.1 during the open-label period among those randomised to givosiran▼, and 1.7 and 9.0, respectively, among those originally randomised to placebo.30 Similarly, mean changes in EuroQol-Visual Analogue Scale (EQ-VAS) scores were +5 and +14 at Months 6 and 24, respectively, among those randomised to givosiran▼, and –1 and +9, respectively, among those originally randomised to placebo.30 Likewise, results from the Porphyria Patient Experience Questionnaire (PPEQ), which was designed for this study, also showed improvements in quality of life, much more so with givosiran▼ versus placebo during the double-blind phase and somewhat more so for those who had received givosiran▼ for 24 months versus those who received placebo for 6 months then givosiran▼ for 18 months.30 These included improvements in “overall satisfaction with treatment”, “convenience of treatment”, “planning for future events”, “traveling >1 day for work or pleasure”, “doing household chores”, “participating in social activities”, “exercising moderately”, and “study drug helping more normal life”. Taken together, these results imply that quality of life improves gradually over time with givosiran▼.
Among all patients combined, adverse events (AEs), serious AEs, and severe AEs occurred in 96%, 30%, and 29% of patients, respectively.30 The most common treatment-related AEs were injection-site reactions (29%), nausea (20%), and fatigue (13%).30 Serious AEs included increased homocysteine, CKD, device breakage, pyrexia, and urinary tract infection (each 2%).30 AEs led to treatment discontinuation in 3% of patients, and there were no deaths by the 24-month interim analysis. Hepatic AEs, which were reported in 18% of patients, were mild to moderate in severity.30 Renal AEs (mostly increased blood creatinine and/or decreased estimated glomerular filtration rate [eGFR]) were reported in 22% of patients, but none led to treatment discontinuation.30 Small decreases in eGFR observed early in therapy stabilised over Months 12–24.30 However, patients on givosiran▼ should be monitored for transaminases and creatinine/eGFR.5,28
Overall, these long-term results from ENVISION show that givosiran▼ treatment provided sustained reductions in attack rates for up to 24 months, with reductions in haemin use and improvements in quality of life. The safety of givosiran▼ during the open-label phase30 was consistent with that during the double-blind phase.29
In summary, AHP remains challenging due to low disease awareness,4 delayed or misdiagnosis,3,7,8 and limited treatment options,1,3 meaning that there is an unmet medical need in patients with AHP. Gastroenterologists can play a vital role in the diagnosis and management of AHP, making them well positioned to identify the hallmark symptoms of AHP and facilitate diagnosis and management. Lastly, the long-term management of AHP with givosiran▼ has been shown to lead to a sustained reduction in porphyria attacks and improve quality of life.30
Questions and Answers
Is urinary ALA and PBG a test that is standardised and available in all university hospital laboratories?
These tests are usually available in university hospitals, but are not necessarily available in primary care or outpatient gastroenterology. These urine tests are very important for the differential diagnosis of abdominal pain, but it is vital to keep the urine sample away from light. If physicians cannot find a laboratory to run these tests, the European Porphyria Network (EPNET) website has a list of diagnostic centres that can run the tests and to which patients with AHP can be referred. Of note, spot urine tests are easier for patients, but many laboratories use 24-hour urine testing. Similarly, quantitative testing is more reliable, but some laboratories only offer qualitative testing.
When should a patient with AHP start receiving givosiran▼?
This largely depends on local reimbursement criteria and the relevant givosiran▼ label.5,28 However, the inclusion criteria for the studies indicate that patients with >2–3 attacks per year who require emergency treatment (e.g., with haemin) should be considered for givosiran▼. Patients who are recurrently hospitalised with abdominal pain are most likely to benefit. However, those with more chronic symptoms may also benefit, so could be considered for givosiran▼ treatment, depending on local reimbursement criteria. Currently, more evidence is needed to ascertain which patients are most likely to benefit from givosiran▼, and how long to continue treatment.
Can you discuss the reasons why patients are misdiagnosed or experience delayed diagnosis?
The main reason is the non-specific nature of AHP symptoms. Various diseases can cause recurrent abdominal pain and neurological symptoms in the CNS, PNS, or ANS (Figure 1). However, recurrent abdominal pain that warrants an ED visit and occurs with neurological symptoms, paraesthesia, muscle weakness, and dark (reddish) urine should raise suspicion for AHP. However, as AHP is very rare, many patients get misdiagnoses of endometriosis or gastrointestinal motility issues. Awareness of AHP therefore needs to be improved, and if imaging, endoscopies, and blood tests all come back negative, porphyria should be considered.
What are the most common symptoms besides abdominal pain, and is abdominal pain also present when patients are not experiencing acute attacks?
Severe abdominal pain is common during acute attacks, but not otherwise. Additional symptoms can include autonomous neuropathy, neuropathic pain, and muscle weakness. Patients may also develop chronic renal insufficiency, liver lesions, hypertension, paraesthesias, and neuropathy. Of note, patients without acute attacks may also develop these chronic problems. Patients with AHP often go through phases of symptom severity (Figure 2), and this should raise suspicion of AHP, especially when pain is combined with neurological manifestations.
How are patients with a lower frequency of attacks managed?
Patients with an acute attack are treated with haemin, analgesics (e.g., morphine), and/or glucose infusions/supplements. Patients are also advised to avoid triggers (e.g., fasting, alcohol, menses, certain medications, and infections). Those with frequent attacks (every month or more) may also require other treatments, e.g., givosiran▼. Regarding medications to avoid, these tend to include those that are metabolised by enzymes that require haem. Drugs can be checked on websites such as the American Porphyria Foundation.
Can you treat pregnant females with givosiran▼?
There are not yet any data on givosiran▼ use during pregnancy, but this will likely become available over time.
How often can you get a false negative diagnosis?
Urine testing for PBG and ALA is very specific, so highly elevated levels can be used to diagnose AHP with a high degree of certainty. Patients with only slightly elevated levels should have the test repeated during an attack. Errors in sample handling (e.g., urine not kept protected from light) could also affect the measurement. False negatives are very unlikely if urine is collected during an attack, but can occur between attacks. In patients with AIP, if the interval between the attack and the test is too long, the cut-off of 3–5 times the upper limit of normal may not be seen.
Would you prescribe givosiran▼ to a patient with kidney disease?
Renal function declines over time in patients with AHP, so kidney disease is somewhat inherent, making it challenging to assess whether there is any renal toxicity related to givosiran▼. Patients with kidney disease can be treated with givosiran▼, but their renal function should be monitored even though there are no data to show that givosiran▼ worsens the normal renal function decline seen in patients with AHP. However, patients with eGFR <30 mL/min/1.73 m2 were excluded from the ENVISION study,29 so data in such patients are not available.
During the ENVISION study, did any patients drop out? If so, what was the reason?
One patient dropped out during the double-blind period as their alanine aminotransferase increased to >8 times the upper limit of normal while on givosiran▼. Some patients had smaller rises in transaminases (approximately 3–5 times the upper limit of normal), which resolved spontaneously over time. However, it is important to monitor the renal and liver function of patients on givosiran▼. Of note, porphyria can cause elevated liver enzymes, with abnormalities visible on ultrasound. It would be interesting to see whether givosiran▼ can help to avoid this.








