Meeting Summary
Patient adherence is a major problem in the treatment of inflammatory bowel disease (IBD). Research has shown that improved patient adherence and outcomes can be achieved if physicians are able to dedicate more time and attention to analysing patients’ feedback on their healthcare. The US Food and Drug Administration (FDA) has defined patient-reported outcomes (PROs) as “any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else.” Such patient reports may include various symptoms that are not obvious or that occur in the absence of an observer; they may describe the frequency and severity of a symptom, and the impact that it has on day-to-day life. They can describe factors such as patient satisfaction, productivity, use of resources, and health-related quality of life (QoL).
Interestingly, PROs do not always correlate with the physician’s view. Evidence has shown that physicians often underestimate the severity of a patient’s illness, report fewer problems than patients, and overestimate the improvements of treatment. In order to improve the value of PROs, physicians must engage patients in their disease management, otherwise known as patient empowerment. Empowerment can improve treatment success.
As it is becoming clearer that empowered and informed patients who are able to have a role in the decision-making have better outcomes, PROs will not only have further impact on the management of patients with IBD but also on health technology assessments and healthcare payer decisions.
Learning Objectives
The aim of this webinar was to review the identified gaps in the knowledge and understanding of the relevance of PROs in the management of patients with IBD. At the end of the webinar, delegates were able to:
- Recognise the benefits of and the need for patient-centric healthcare
- Appreciate the relevance of PROs in the management of patients with long-term IBD
- Identify which PRO measures are the most useful in the management of patients with IBD
- Understand how the systematic use of information from PRO measures leads to better communication and decision-making between physicians and patients, and how this improves patient satisfaction with care and self-management
Inflammatory Bowel Disease Overview and Treatment
IBD includes Crohn’s disease and ulcerative colitis (UC), inflammatory conditions of the bowel which both require close management to control symptoms. However, patient adherence remains a major barrier to effective treatment. The treatments for IBD currently recommended by the European Crohn’s and Colitis Organisation (ECCO) guidelines1 include 5-aminosalicylic acid (5-ASA) derivatives such as mesalazine and sulfasalazine, supportive therapy such as loperamide, antibiotics/probiotics, parenteral and enteral nutritional therapy, long-term steroid treatment, and immunomodulators and biologics such as azathioprine, infliximab, and adalimumab. Surgery may also be an option if there is development of strictures, neoplasia, or refractory disease, or in the presence of treatment-related side effects.
Treatment Goal Evolution in Inflammatory Bowel Disease
Over time, treatment goals have evolved from symptom control to disease control (endoscopic and biochemical control). Initially, the aim was for rapid, clinical control of symptoms which indicated induction of remission, and subsequent endoscopic and biochemical control which indicated complete remission. Now the aim of treatment is to allow patients to live a normal life without disability and without being affected by the side effects of their disease. An optimal management plan then includes maintenance of remission, cancer prevention, enhancement of mucosal healing and histological remission, improvement in patients’ QoL, including their ability to socialise and go to work, avoidance of long-term steroids, and the prevention of growth retardation in children, whilst adopting a safe therapeutic strategy.
Patient-Centric Healthcare
What Can the Patient Do to Meet Their Inflammatory Bowel Disease Treatment Goals?
In order to detect flares of IBD early and to minimise complications, patients should record the key elements of the partial Mayo-Score on a regular basis, including bowel movements and rectal bleeding. They should also be aware of abdominal or perianal pain, extra-intestinal manifestations, and fatigue. Patient knowledge of potential treatment side effects (including increased bleeding, fever, weight loss, and lymph node swelling) or treatment failure allows early identification and time to address these issues before they become more serious. Although some tools are available online, currently there is no validated guidance for communicating the risks associated with treatments to our patients.
What Can Physicians Do?
When assessing IBD in the past, physicians would focus on endoscopy results. However, this did not consider any symptoms or feelings the patient may be experiencing. It is now known that a combination of symptom control with improved endoscopy and biochemistry results can lead to a more complete and prolonged remission, allowing the patient to live a normal life without disability and disease-related side effects. Research has shown that when physicians provided more attention to patient feedback on their healthcare, improved treatment adherence and patient outcomes were achieved.
Defining Patient-Reported Outcomes: European Medicines Agency and The US Food and Drug Administration’s Definitions
The European Medicines Agency (EMA) has defined a PRO as “any outcome evaluated directly by the patient themselves and based on the patient’s perception of a disease and its treatment,”2 and described it as an umbrella term which covers both single and multi-dimension measures of symptoms, health-related QoL, health status, adherence to treatment, and satisfaction with treatment. The FDA has also described a PRO as “any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else.”3 The outcome can be measured in absolute terms (such as severity of symptoms and signs or state of a disease) or as a change from a previous measure.3
PROs may include various clinical symptoms that are not obvious to an observer (such as fatigue or headache), psychological symptoms, or symptoms that occur in the absence of an observer (such as sleep disturbances).4 Patients may also provide details of the frequency and severity of their symptoms and the impact their disease has on their QoL. PROs may include an assessment of the patient’s feelings towards their disease and treatment.4
How Can Patient-Reported Outcomes Help in the Treatment of Inflammatory Bowel Disease?
In IBD, the perspective of the patient does not always correlate with that of the physician, which ultimately has an impact on patient care.5,6 Research has shown that overall, physicians report fewer symptoms than patients; they underestimate the severity of the illness and overestimate treatment outcomes.6,7
An analysis of the different perceptions of UC severity between patients and physicians in multiple countries found that, in the UK for example, 33% of patients compared with 52% of physicians described their disease severity as mild.6 Similarly in Ireland, 36% of patients described their UC as severe, compared with 16% of physicians.6 The same study also analysed the different perceptions in the number of UC flares between patients and their physicians. It found that in the UK and Canada for example, the physician-estimated number of flares was half of that reported by patients (6.5 versus 2.6 and 6.2 versus 3.3, respectively, over a 12-month period).6 Perceptions regarding the incidence of UC symptoms also varied between patients and physicians, for example 37% of patients from France reporting blood in their stools compared with only 22% of physicians reporting this.6
PROs also often represent the things that are most important to a patient regarding their condition and care.8 A study using the Patient-Reported Outcomes Measurement Information System (PROMIS) found that the PROs of depression, anxiety, fatigue, sleep disturbance, pain interference, and satisfaction with their social role were worse in IBD patients than the general population, and similar to those reported for other chronic diseases.9 One study in Crohn’s disease using a PRO-2 assessment scale (including stool frequency and pain) found that effect estimates were similar to using a more comprehensive measure of disease activity (Crohn’s Disease Activity Index [CDAI]).10 A similar study in UC reported corresponding results with a two-item PRO of rectal bleeding and stool frequency.11 Such two-item PRO scales are frequently used in IBD although there is a need to allow for regional and cultural differences; further development and assessment of PROs in IBD would be valuable to provide more guidance for physicians and to allow subsequent inclusion in treatment guidelines.
How Can we Measure and Assess Patient-Reported Outcomes?
All clinicians informally request PROs in the assessment of patients alongside the formal clinical tests by asking questions such as: ‘how many bowel movements have you had in the past 2 weeks?’, ‘have you seen any blood?’, and ‘have you experienced any pain?’. The use of a PRO instrument can significantly aid this conversation, ensure all aspects of the patient view is assessed, and allow comparison with previous reports. A PRO instrument should be valid in that it is reliable, able to provide the same results on separate occasions, responsive, able to detect small changes within or between groups, feasible, easy to administer considering budget and time constraints, and finally, it must be documented in a PRO evidence dossier which summarises the PRO assessment strategy, the qualities of the PRO instrument, and the results.12
When using PROs, it is important to engage and empower patients in the management of their disease. Patient empowerment is very important in improving adherence and outcomes as it puts the patient at the centre of their care. It enables patients to take control of their own health, create awareness of early treatment failure, improve their disease management (including preventing complications), and allows them to assess their QoL. An empowered patient can better understand their condition and treatment, can participate in decision making, make informed choices with the healthcare professional, and can actively seek care when they need it. To enable patient empowerment, it is important that there is two-way communication with patients and that they can speak a common language with their physician.
When making decisions about medication, patient involvement is particularly important and may help identify patients who may not adhere to their medication. Increasing patient knowledge and understanding of their medication may work to improve their adherence.13 A study analysing patients’ suggestions to improve their current treatment found that 54% of patients would like more information regarding new medications and 50% would value a closer collaboration with their physician.14
A Patient Case Study
Patient empowerment and the use of PROs is demonstrated with Anna (fictitious name), a 29-year-old teacher diagnosed with ulcerative pancolitis 7 years ago. She was initially treated with 3.2 g/day mesalazine and once in remission, was maintained with 2.4 g/day mesalazine. Anna suffered from a severe flare 1 year ago which was treated with systemic corticosteroids. She then achieved rapid remission, maintained with mesalazine as before. However, she was now experiencing a new flare with four bowel movements per day, sometimes bloody stools, urgency, and abdominal pain. By using a PRO instrument, it was identified that Anna was concerned about diarrhoea, abdominal pain, and bloody stools. She also had questions regarding the safety of her treatment and the side effects caused by steroids, immunosuppressants, and biologics. For Anna, or any patients with extensive mild-to-moderate colitis, the ECCO guidelines advise treatment with mesalazine >2 g per day plus topical mesalazine.15 Oral 5-ASA is first-line maintenance treatment in patients responding to 5-ASA or steroids (oral or rectal).
In addition to increased bowel movements, pain is a common and important symptom of IBD for patients. A study into the distribution of pain in IBD patients found that pain was experienced mainly in the abdomen, rectum, back, and joints, with no significant variation between males and females.16 Anna additionally noted that she had concerns regarding abdominal and joint pain, as well as the possible side effects of fatigue and driving/work restrictions. The ECCO guidelines do not make any specific treatment recommendations for pain described as abdominal and with extra-intestinal manifestations (including arthralgia, joints, and uveitis) or as a complication of a patient’s medication (such as pancreatitis),16 although patients are advised to avoid non-steroidal anti-inflammatory drugs. Combined therapy with oral mesalazine 4.8 g/day and 1 g mesalazine enema was initiated for Anna. This induced rapid remission and she reverted to remission maintenance therapy with mesalazine 2.4 g/day.
Medical Management of Inflammatory Bowel Disease During Pregnancy
Mothers with active IBD at conception and during pregnancy have a higher risk of spontaneous abortions, birth malformations, preterm birth, low birth weight, and small babies for gestational age compared with mothers in disease remission.15 Anna requested advice on the risks of IBD and its treatment related to pregnancy. Specifically, she was worried about how her IBD and its treatment could affect her unborn child and was concerned about the effect of pregnancy on her own health. The ECCO guidelines state that disease activity at conception is an important risk factor for adverse pregnancy outcomes and increases the risk of persistent activity during pregnancy.15 If conception occurs at a time of quiescent disease, the risk of relapse is the same as in non-pregnant women.
Several studies have assessed the outcome of pregnancies in patients with IBD and whether outcomes are influenced by disease activity (Table 1). These results show that the outcome of pregnancy in patients with IBD is highly dependent on the disease activity throughout the pregnancy. Therefore, if possible, pregnancy should be planned for an inactive phase of IBD.
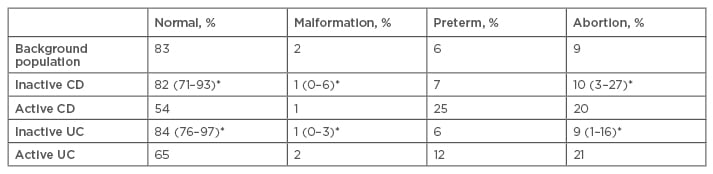
Table 1: Outcome of pregnancies in patients with inflammatory bowel disease can be influenced by disease activity during the gestational period.15,17-22
*Percentage range across individual studies.
UC: ulcerative colitis; CD: Crohn’s disease.
Standard IBD medications such as sulfasalazine, 5-ASA derivatives, steroids, and budesonide seem to be generally safe to use during pregnancy and breastfeeding, with the exceptions of methotrexate and thalidomide.15 Fetal exposure to thiopurines is not associated with increased risk of infections in Year 1. However, risk of infection with anti-tumour necrosis factor agents alone or in combination with immunomodulators is controversial. Furthermore, the risk of spontaneous abortions and fetal abnormalities are not significantly increased with basic IBD medications.17 However, there is an increased risk of preterm births and reduced weight at birth;17 there is no indication for therapeutic abortion.18 Active IBD can be treated with 5-ASA derivatives, budesonide, and corticosteroids as in non-pregnant patients. There should be paediatric surveillance in cases of high-dose preterm steroid treatment. The safety of some IBD medications in pregnancy has been summarised in Table 2.
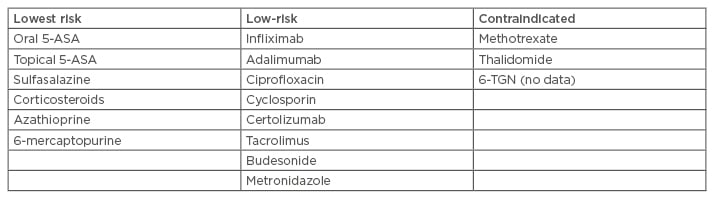
Table 2: Safety of inflammatory bowel disease drugs in pregnancy (the European Crohn’s and Colitis Organisation [ECCO] rating).15,17,18
5-ASA: 5-aminosalicylic acid; 6-TGN: 6-thioguanine nucleotide.
Anna became pregnant and continued mesalazine 2.4 g/day. She suffered an acute flare in Week 22 of her pregnancy. For >2 weeks she had six bowel movements per day (sometimes bloody) and also experienced nocturnal bowel movements. She had mild abdominal pain, haemoglobin 10.5 g/dL, leukocytes 14.5 /nl, C-reactive protein 20 mg/L, and calprotectin 650 μg/g. With the flare and additional treatment needed, with anxiety about her IBD, the treatments and risks to her unborn baby increased. After a clear discussion with Anna about the data on safety in pregnancy, she agreed to the treatment plan. 5-ASA therapy was optimised, increasing oral mesalazine to 4.8 g/day and adding a mesalazine enema of 1 g/day for 2 weeks. After 10 days Anna went into clinical remission and continued oral mesalazine 4.8 g/day. Her pregnancy continued normally and Anna delivered a healthy baby boy 3 days prior to her due date.
Summary and Conclusions
As the knowledge and understanding of IBD increases, the treatment goals are changing. PROs will have a subsequent impact on the management of IBD patients and the decision-making process for health technology assessments and healthcare payers. Empowered and informed patients are able to participate more meaningfully in decision making and monitor their disease course.
The ECCO guidelines do not specifically include information relating to the use of PROs.23 The key aims of medical intervention in IBD are steroid-free remission and no harm by therapeutic strategies. The optimal early treatment in IBD is the safest treatment that will lead to steroid-free deep remission (clinical, biological, and intestinal) within 1 year following the diagnosis. The guidelines recommend that treatment should be selected with the patient and consider their personal preference.23 Thus, this emphasises the future importance of PROs in improving the care and treatment of patients with IBD.
Click here to view the full webinar.








