Meeting Summary
Prof Gralnek presented a clinical case on the management of gastrointestinal bleeding (GIB) as a result of Helicobacter pylori infection, and the role of intravenous (IV) ferric carboxymaltose (FCM) as a treatment option for iron deficiency anaemia (IDA) was discussed. IV iron is suitable for patients who have intolerance or limited or no response to oral iron, haemoglobin (Hb) <10 g/dL, or Hb >10 g/dL with cardiovascular or respiratory comorbidities. Prof Gralnek stressed that IDA is common, often underdiagnosed and undertreated, and that the choice between oral or IV iron therapy depends on the degree of anaemia, presence of inflammation, and adherence to oral iron therapy. The main objective of iron treatment is to normalise Hb and iron parameters, and gastroenterologists need to be more aware of anaemia beyond the acute GIB episodes.
Prof Lanas presented a clinical case on the management of patients taking anticoagulants (AC) or antithrombotics (AT) who have anaemia due to GIB, and highlighted challenges associated with reducing the risk of bleeds while avoiding thrombotic events. Prof Lanas highlighted clinical dilemmas arising from stopping, restarting, and switching AC in patients with anaemia and GIB, as well as Hb management at discharge. He also stressed that GIB, and especially anaemia or iron storage depletion, are frequently encountered in patients taking AT, and may have a direct impact on mortality, morbidity, and quality of life (QoL). Anaemia and iron deficiency affect mortality, recovery, and QoL in patients who need a rapid restoration of Hb levels and iron stores to decrease the risk of cardiovascular events. Prof Lanas concluded by explaining that FCM therapy has a favourable safety profile, and is more effective, faster, and cost-effective compared to oral iron therapy, and therefore represents a good therapeutic option for anaemic GIB patients with elevated risk of thrombosis.
Clinical Case: Acute Gastrointestinal Bleed in a Helicobacter Pylori Positive Patient
Professor Ian M. Gralnek
Case Presentation
Prof Gralnek opened the symposium with a clinical case: a 52-year old woman presented with dizziness and explained that she had had bloody stools (melena) for 48 hours. A year prior to this, she had seen her doctor for indigestion (dyspepsia), and a urea breath test returned positive, indicating that she had an active H. pylori infection, for which she was treated with antibiotics. She was then lost to follow-up. In the emergency department, the patient said that she had not been taking any long-term medications. She had a normal blood pressure (102/64 mmHg), but her pulse was moderately tachycardic (112 beats per minute). Upon palpation, her abdomen was soft, and she only complained of mild epigastric pain. A rectal exam revealed melena, and she was anaemic (Hb: 8.2 g/dL). One year prior, her Hb was 10.3 g/dL, iron 12.0 µM/L, ferritin 6.0 ng/mL, and transferrin saturation index 9.0%. Gastroscopy revealed a small bleeding ulcer, which was treated with clipping to stop the GIB. A rapid urease test at endoscopy revealed a persistent H. pylori infection. The patient was transfused with 2 units of red blood cells (RBC), which increased her Hb to 9.6 g/dL. Next, she was treated with IV proton pump inhibitor (PPI), and re-treated with antibiotics for H. pylori. Finally, she was given oral PPI and discharged home.
At a clinic follow-up 2 weeks later, the patient was feeling better, and her Hb was 9.1 g/dL. She mentioned that she had always had anaemia and low iron, and she has had heavy menses since she was a teenager. She also told the treating physician that she does not like oral iron supplements because they give her abdominal pain and constipation. Furthermore, she has no family history of GI malignancies, and has had no prior colonoscopy for colorectal cancer screening. At a clinic follow-up 8 weeks later, a colonoscopy to the terminal ileum was negative, as was a follow-up post-gastric ulcer gastroscopy. Duodenal and gastric biopsies were also negative, and her repeat Hb was 9.4 g/dL.
Prof Gralnek concluded the case presentation by highlighting the importance of investigating the cause of the underlying IDA and the need to consider treating underlying IDA on top of acute GIB.
What the Science Tells Us about How to Treat Gastrointestinal Bleeding in Patients with Underlying Iron Deficiency Anaemia
A retrospective study from Denmark identified that 80% of acute nonvariceal upper GI haemorrhage patients were discharged with anaemia, and that only 16% had oral iron recommended at discharge. This may be partly because no standardised follow-up protocols were in place for patients with anaemia at the time.1 It has since become evident that the gastroenterologist must not only treat the acute GIB event but also the underlying anaemia and iron deficiency. Anaemia is associated with disability, poorer physical performance, and lower muscle strength,2 and a prospective study on women aged 65 years or older reported that the 5-year all-cause mortality increased when Hb was below the World Health Organization (WHO) low-normal cutoff of 12 g/dL.3
Little attention has been paid to the health-related QoL of patients with GIB because this aspect requires contact to be maintained with patients and actions to be implemented after the hospital discharge. The appropriate management of anaemia and iron deficiency has the potential to directly affect the QoL and performance of most patients. Implications for physical health, mobility, performance of daily activities, pain, discomfort, anxiety, and depression following hospital admission and discharge have not been adequately investigated but are as important to patients and families as the bleeding itself. These aspects need to be featured within the physician’s agenda when considering the appropriate management of patients with GIB.4
Oral iron preparations are the mainstay for the treatment or prevention of IDA, and numerous oral preparations and dosing regimens are available.5 IV iron preparations with improved toxicity profiles have been used for cases in which rapid therapy was useful in reducing the need for RBC transfusion.6 As a general rule, iron supplementation therapy should be continued until the anaemia is resolved and the iron stores are replenished.7,8 Oral administration of iron sulphate is viewed as the first-line treatment for IDA patients; however, oral iron shows substantial limitations for patients with GI disorders because of insufficient absorption, slow course of action, and severe GI side effects that may exacerbate existing symptoms.9 Due to this intolerance, oral iron treatment is discontinued in up to 50% of IDA patients.10
Ferric carboxymaltose (FCM) is one of several IV iron formulations11 available and was first approved in Europe in 2007.12 The FCM formulation consists of a complex carbohydrate shell that tightly binds the elemental iron, which allows a large dose of supplemental iron to be administered IV in a short time period.12,13 In a 42-day clinical trial (N=71 patients) comparing FCM with oral iron (ferrous sulphate), FCM was more efficacious and faster to normalise Hb levels (Figure 1a) and transferrin saturation index (Figure 1b) than oral iron.9 No treatment-related adverse events, withdrawals, or dose reductions were reported for the FCM group, whereas treatment-related adverse events (mainly constipation) were reported by 30% of oral iron treated-patients.
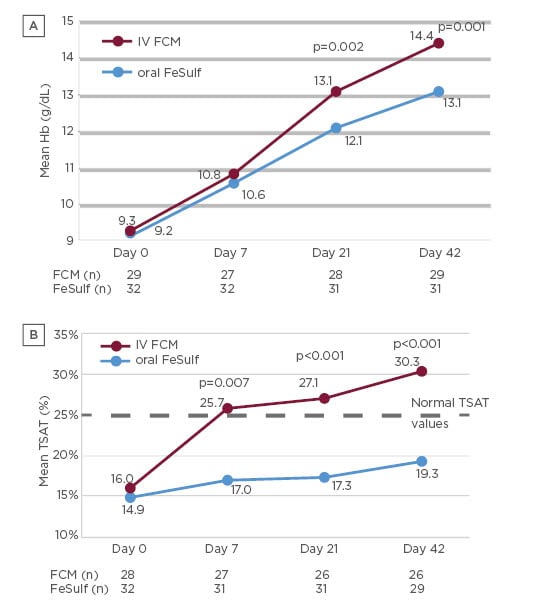
Figure 1: Mean Hb levels (a) and mean transferrin saturation index (b) of patients treated with IV FCM or oral iron (FeSulf).9
FCM: ferric carboxymaltose; FeSulf: ferrous sulphate; Hb: haemoglobin; IV: intravenous; TSAT: transferrin saturation index.
At Day 42, 77% of FCM-treated patients repleted their iron deposits compared with only 24% of patients treated with oral iron, with a significant difference (p=0.04) between the two treatments observed already at Day 7. Patients treated with FCM also had a significantly better (p=0.02) subjective state of health (measured using the EuroQol-visual analogue scale) versus patients treated with oral iron.9 A summary of advantages and limitations of oral or IV iron replacement therapies are summarised in Table 1.14
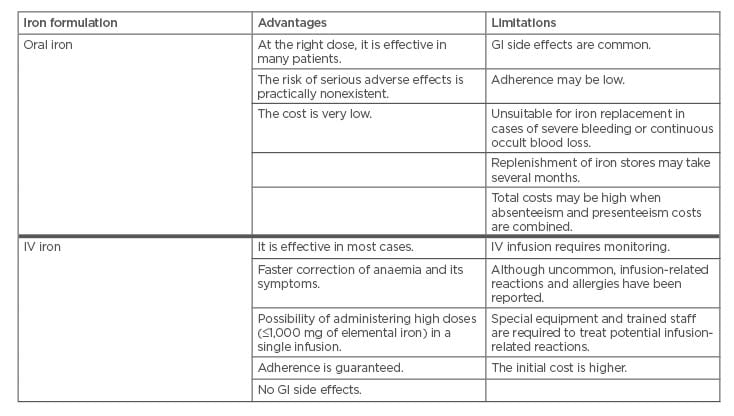
Table 1: Advantages and limitations of oral iron and IV iron formulations. Courtesy from Spanish Association of Gastroenterology (AEG).14
GI: gastrointestinal; IV: intravenous.
Prof Gralnek argued that IV iron is suitable for patients with intolerance or limited or no response to oral iron, a Hb <10 g/dL, or with a Hb >10 g/dL in conjunction with cardiovascular or respiratory comorbidities. IV iron therapy has a favourable safety profile, as reported by a systematic review and meta-analysis of 103 trials. This study concluded that although IV iron is associated with infusion reactions, it is also associated with reduced rates of GI adverse events, and it is not associated with an increased risk of serious adverse events or infections.15
Furthermore, IV iron is cost-effective compared to other anaemia therapies such as RBC transfusion,16-18 which is a leading indication for acute upper GI bleeding, although the optimal Hb thresholds for transfusion are poorly defined. A meta-analysis of five randomised trials found that restrictive RBC transfusion results in lower all-cause mortality and lower re-bleeding compared with liberal RBC transfusion.19 Additionally, FCM therapy in patients with chronic GIB reduces the need for RBC transfusions and improves Hb and iron indices.17
Prof Gralnek concluded his case-based presentation on IDA in patients with GIB by stressing that IDA is common, often underdiagnosed, and undertreated. The decisions on whether to treat with RBC transfusion should be made individually, depending on the origin and extent of the bleeding and the existence of patient comorbidities. The choice between oral versus IV iron therapy will depend on multiple factors such as the degree of anaemia, presence of inflammation, and adherence to oral iron therapy. The main objective of iron treatment is to normalise Hb and iron parameters, and gastroenterologists need to be more aware of anaemia beyond the acute GIB episode. Furthermore, future GIB guidelines should consider recommendations on iron therapy.
Acute Gastrointestinal Bleeding in Patients Taking Anticoagulants or Antiplatelets
Professor Angel Lanas
Case Presentation
Prof Lanas opened his presentation by introducing a typical clinical case: a 74-year old woman with Type 2 diabetes mellitus, hypertension, and atrial fibrillation. She was taking metformin, candesartan, and warfarin, and had noticed the presence of red blood in the rectum for several days and had been feeling weak. Her primary care physician detected Hb 6 g/dL and an international normalised ratio (INR) of 4. The patient was hospitalised and diagnosed with colonic diverticular bleeding. Prof Lanas highlighted important clinical considerations associated with stopping the AC, and if so, for how long. Other considerations included whether to change the AC to a different type if resumed, as well as challenges associated with Hb management at discharge in anaemic GIB patients that are taking AC or antiplatelet (AP) drugs.
What the Science Tells Us About How to Treat Anemia in Acute Gastrointestinal Bleeding Patients Taking Anticoagulant or Antiplatelet Drugs
More than 5,700 severe bleeding episodes linked to AC occur in Spain every year. The largest proportion of these bleeding events linked to AC therapy due to atrial fibrillation is digestive system bleeds (43.6%), followed by brain bleeds (30.6%), and urogenital bleeds (14.5%).20,21 Patients with IDA of unknown aetiology are usually referred to a gastroenterologist because GI conditions are likely to be the cause of the bleed.22 Nonvariceal upper GIB is a type of bleeding that develops in the proximal duodenum, stomach, or oesophagus. Peptic ulcers, caused by H. pylori infection or use of nonsteroid anti-inflammatory drugs (NSAID) and low-dose aspirin, are the most common causes of nonvariceal upper GIB.4
Prof Lanas pointed out that in nonvariceal upper GIB in patients taking vitamin K antagonists such as warfarin, it is important to determine the INR once the vitamin K antagonist has been stopped. If the INR is <2.5, the patient should be referred for endoscopy, and if the INR is >2.5, the treatment plan depends on whether or not the patient is haemodynamically compromised.4 The timing for when to restart AC therapy depends on the clinical context. An observational cohort study of patients who developed GI bleeding while on AP or AC therapy found that with AP/AC, the cardiovascular risk is reduced at the expense of an increased risk of recurrent GIB.23 There is no firm evidence for when AP or AC therapy should be resumed. European Society of Gastrointestinal Endoscopy (ESGE) guidelines recommend restarting AC therapy following nonvariceal upper GIB in patients with an indication for long-term anticoagulation.24 The timing for resumption of anticoagulation should be assessed on a patient-by-patient basis: within 7–15 days following the bleeding event for most patients, and within the first 7 days for patients at high thrombotic risk.24 Importantly, early resumption of AT therapy is not associated with poorer outcomes, and appears beneficial.23
Should the Gastroenterologist be Concerned with the Patient’s Haemoglobin Levels at Discharge?
In the illustrative clinical case, the patient received three units of RBC and was discharged after the GIB stopped spontaneously. She is now treated with a new oral direct AC and oral iron, and her Hb at discharge was 9.5 g/dL. A frequent clinical scenario after an acute GIB event at hospital discharge is that the GIB is controlled, the patient has comorbidities, and remains anaemic (Hb between 7 and 10 g/dL) either after receiving blood transfusion or no blood at all. In the clinical case, the patient felt weak and physically limited, and was prescribed oral iron, without clear instructions given to their primary care physician. Many patients also need aspirin or AC, as in the case presented by Prof Lanas, which puts them at risk of rebleeding or maintaining occult blood loss. Importantly, there are data suggesting that patients leaving the hospital with low Hb have an increased risk of death,25 but patients who receive IV iron are less likely to reattend hospital within 30 days, are more likely to reattend electively, and tend to have a shorter length of stay in hospital compared with patients who receive oral iron therapy.26 Treatment with FCM for acute GIB is associated with a good erythropoietic response and anaemia correction after hospitalisation, even in severe episodes or when transfusion is needed. Favourable outcomes (Hb increase of 3–6 g/dL) of FCM treatment were observed also in patients that were aged ≥75 years, had a high Charlson Comorbidity Index ≥3, or who had low Hb (≤10 g/dL) at admission. Additionally, FCM has a favourable safety profile, is well-tolerated, and may support a restrictive blood transfusion policy.27
Prof Lanas brought a question to the audience: What Happens to those Patients that did not have a Previous Gastrointestinal Bleed but were Receiving Drugs Potentially Toxic for the Gastrointestinal Tract, such as Aspirin, Antiplatelet, Anticoagulant, or Nonsteroidal Anti-Inflammatory Drugs?
Anaemia and iron deficiency are common in patients with cardiovascular disease,28 and a retrospective observational cohort study of patients who started dual AP therapy (DAPT, i.e., clopidogrel plus aspirin) after percutaneous coronary intervention reported that anaemia and iron deficiency were the most commonly reported GI events. After 1 year of follow-up, almost 50.0% of patients taking dual AP therapy developed anaemia or iron deficiency and only 3.6% developed a major GI event. This data is important because most of the patients (90%) were receiving PPI, even though a majority (72%) of the lesions responsible for the anaemia were found in the lower GI tract.29 GIB have also been associated with NSAID therapies. The CONDOR study investigated the risk of GIB in patients with osteoarthritis or rheumatoid arthritis that were treated with either celecoxib (a cyclo-oxygenase-2-selective NSAID) or diclofenac (a nonselective NSAID) plus a PPI, with clinically significant GI events through the GI tract as primary endpoint. The authors concluded that the risk of developing clinically significant GI events was lower in patients treated with a cyclo-oxygenase -2-selective NSAID than in patients treated with a nonselective NSAID plus a PPI. Importantly, 92 of the 101 reported GI events were not major bleeds, but anaemia cases with a Hb decrease of ≥2 g/dL due to GI origin bleeds.30 This Hb decrease may be countered by FCM therapy, because patients with chronic GIB that are treated with FCM have a reduced need of RBC transfusion and improved Hb and iron parameters.17
Conclusion
In summary, upper and lower GIB, and especially anaemia or iron storage depletion, are frequent in patients taking AT agents, and occur in patients with comorbidities such as cardiovascular disease, which have a direct impact on mortality, morbidity, and QoL. Anaemia after hospital discharge is common in patients who have developed a GIB event, and in patients taking AP or AC drugs. These patients are at the highest risk of remaining anaemic, since most will need to maintain their medication, and many will develop anaemia without presenting with a major GIB event. Anaemia and iron deficiency affect mortality, recovery, and QoL in patients who need a rapid restoration of haemoglobin levels and iron stores to decrease the risk of new and recurrent cardiovascular events. FCM therapy is more effective, faster, has a favourable safety profile, and is cost-effective compared to oral iron therapy, and represents a good therapeutic option for this type of patient.








