Meeting Summary
Prof Ghosh presented data from the UNITI studies exploring ustekinumab in primary or secondary nonresponders to TNF agonists (UNITI-1) and conventional therapy failures (UNITI-2). The data demonstrate that ustekinumab shows higher efficacy in patients who have failed conventional therapy compared to those who have failed anti-TNF therapy. Further sub-studies showed similar efficacy for ustekinumab 90 mg every 8 weeks (q8w) and ustekinumab 90 mg every 12 weeks (q12w) subcutaneous (SC) regimens, except for in patients with high inflammatory burdens, who did better with q8w regimens. No new safety signals were identified for ustekinumab between Week 96 and Week 156, with overall rates of adverse events and serious adverse events being comparable to placebo. Rates of antibody formation remained low.
Dr Raine described two case studies involving Crohn’s disease patients treated with ustekinumab. The first case described a female patient with luminal Crohn’s disease who had secondary nonresponse to an anti-TNF with signs of intestinal and systemic inflammation.
The second case considered a patient with bio-naïve luminal Crohn’s disease who had a previous history of opportunistic infections (coughs, colds, and recurrent herpes simplex).
Prof Armuzzi presented the results of the induction part of the UNIFI study, which randomised patients with moderate-to-severe ulcerative colitis (UC) to placebo, ustekinumab 130 mg, or a weight-tiered ustekinumab dose (˜6 mg/kg). Results showed clinical remission at Week 8 was 5.3% for placebo, 15.6% for ustekinumab 130 mg intravenous (IV) (p<0.001), and 15.5% for ustekinumab at ˜6 mg/kg IV (p<0.001). Furthermore, ustekinumab IV induced clinical response and endoscopic and mucosal healing, improved health related quality of life, and had an adverse event profile consistent with known safety profiles.
Introduction
The learning objectives of the symposium were for participants to describe recent data regarding therapies targeting the IL-12/23 pathway and to explore the impact emerging treatment options are having on the inflammatory bowel disease (IBD) therapeutic landscape. Overall, the presentations were designed to enable the audience to analyse the pros and cons of different treatments and learn how they translate into clinical practice.
Current Developments in Inflammatory Bowel Disease: Long-Term Maintenance with Ustekinumab in Crohn’s Disease
Professor Subrata Ghosh
The UNITI-1 and UNITI-2 studies explored ustekinumab, a monoclonal antibody to the p40 subunit of IL-12 and IL-23, in two Crohn’s disease populations: UNITI-1 considered primary or secondary nonresponders to TNF antagonists, while UNITI-2 considered conventional therapy failures.1
For induction, patients were randomised to a single IV dose of ustekinumab or placebo.1 At Week 8, all UNITI-1 and UNITI-2 responders to IV therapy participated in the maintenance trial (IM-UNITI), where they were randomised to placebo or SC maintenance injections of ustekinumab 90 mg, either q8w or q12w.2 At Week 44, responding patients were eligible for IM-UNITI long-term extension, which evaluated the safety and efficacy of SC ustekinumab for up to 5 years from induction (this is the primary randomised population).2
A post-hoc analysis of IM-UNITI demonstrated that patients with high inflammatory burden do better taking ustekinumab q8w than q12w.3 The analysis stratifying patients in clinical remission at Week 44 according to baseline faecal calprotectin (fCal), showed that with higher baseline fCal (>250 µg/g) clinical remission was achieved in 56.4% of those receiving ustekinumab 90 mg q8w (p=0.002 versus placebo) versus 46.1% of those receiving ustekinumab 90 mg q12w (p=0.065 versus placebo).3 No such difference was found for lower baseline fCal (≤250 µg/g), where 53.0% of patients treated with ustekinumab 90 mg q8w achieved clinical remission (p=0.085 versus placebo), compared with 50.0% treated with ustekinumab 90 mg q12w (p=0.296 versus placebo).3
A study categorising trough levels of ustekinumab into quartiles demonstrated that when compared with quartile 1 (≤0.5 µg/mL), quartile 2 (>0.5 to ≤1.4 µg/mL), quartile 3 (>1.4 to ≤2.7 µg/mL), and quartile 4 (>2.7 µg/mL) provided improved endoscopic response (p=0.006) and remission (p=0.054).4 Furthermore, the study showed that ustekinumab 90 mg q8w resulted in more patients with average trough levels in quartiles 3 and 4, while ustekinumab q12w resulted in more patients with average trough levels in quartiles 1 and 2.4
In the IM-UNITI long-term extension, Week 44 IM-UNITI completers could enter long-term extension without further dose adjustment, although a one-time dose adjustment to 90 mg q8w was allowed for loss of response between Weeks 8 and 32.2 Following unblinding, placebo patients were discontinued.2
Additionally, IM-UNITI included a nonrandomised population of induction patients with no clinical response to ustekinumab IV who received ustekinumab 90 mg q8w SC, and induction patients with no clinical response with IV placebo who received ustekinumab IV followed by ustekinumab 90 mg q12w SC.2 Altogether, 56% (718/1,281) of all patients starting IM-UNITI continued into the long-term extension (Figure 1).2
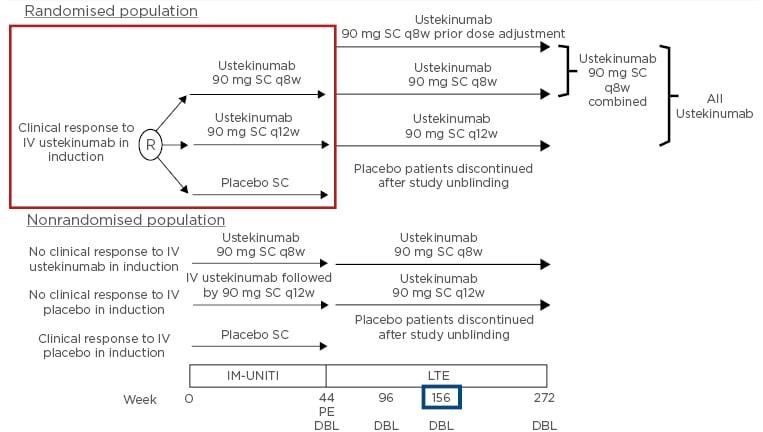
Figure 1: Study design: IM-UNITI long-term extension.
DBL: database lock; IV: intravenous; LTE: long-term extension; PE: primary endpoint; q8w: every 8 weeks; q12w: every 12 weeks; R: randomised; SC: subcutaneous.
Adapted from Sandborn WJ et al.2
An abstract of IM-UNITI data to 2 years shows that, for patients receiving continuous ustekinumab 90 mg q8w, there was no clinical remission difference between patients who received concomitant immunomodulators at baseline and those who did not.5 Furthermore, the study presented at ECCO 2019 showed no difference in serum ustekinumab between the two groups.5
Three-year results for IM-UNITI long-term extension showed that, among ustekinumab-treated patients, remission rates at Week 44 were 84.1% for ustekinumab 90 mg q8w and 77.4% for ustekinumab 90 mg q12w; at Week 92, remission rates were 74.4% for ustekinumab 90 mg q8w and 72.6% for ustekinumab 90 mg q12w; and at Week 152, remission rates were 69.5% for ustekinumab 90 mg q8w and 61.9% for ustekinumab 90 mg q12w.2 Such data demonstrates that ustekimumab 90 mg q8w and q12w perform in a similar way.2
In an intention-to-treat analysis, 38.0% of stekinumab 90 mg q12w and 43.0% of ustekinumab 90 mg q8w induction responders were in remission at Week 152.2 Amongst TNF-α inhibitor-naïve patients at Week 152, 53.8% of ustekinumab 90 mg q8w patients were in remission, compared to 50.9% of ustekinumab 90 mg q12w patients.2
However, a markedly different picture emerges when UNITI-1 patients who failed TNF antagonists are considered separately. At Week 152, analysis of UNITI-1 showed that 59.3% of ustekinumab 90 mg q8w patients were in remission, compared to 43.8% of ustekinumab 90 mg q12w patients. For UNITI-2 subjects (i.e., those who failed conventional therapy) no such difference emerged: remission rates were 74.5% for ustekinumab 90mg q8w versus 73.1% for ustekinumab 90 mg q12w.2 The analysis further demonstrates that ustekinumab achieved greater clinical remission in patients who previously failed conventional therapy compared to those who failedanti-TNF therapy.2
The IM-UNITI long-term extension study showed antibody levels to be low, with antibodies to ustekinumab through Week 156 occurring in 4.6% (11/237) of patients randomised to ustekinumab, 4.0% (8/202) of patients receiving continuous ustekinumab, and 2.4% (2/82) of patients on ustekinumab q8w.2
No new safety signals were identified between Weeks 96 and 156, with overall adverse and serious adverse events being comparable to placebo.2 Serious infection rates per 100 patient-years follow-up were 3.97 for placebo, 5.98 for ustekinumab 90 mg q12w, and 3.13 for ustekinumab 90 mg q8w.2
The case of a 38-year-old man with ileocolonic Crohn’s disease and a history of nonmelanoma skin cancer was reviewed. The patient had joint pain, plaque psoriasis, and deep ulcers in the ileum and caecum. He was refractory to corticosteroids, and his fCal and C-reactive protein (CRP) levels presented at 376 μg/g and 32 mg/L, respectively.
Considering ustekinumab maintenance therapy, 44.0% of the audience selected ustekinumab 90 mg q8w as the most appropriate treatment, 27.4% selected ustekinumab 90 mg q8w de-escalated to 90 mg q12w, 19.4% selected ustekinumab 90 mg q12w, 4.6% selected ustekinumab 90 mg q12w plus azathioprine, and 4.6% selected ustekinumab 90 mg q8w plus azathioprine.
In conclusion, Week 44 IM-UNITI data show that ustekinumab delivers better efficacy in patients who have failed conventional therapy than in those who have failed anti-TNF therapy. Similar efficacy was achieved for q8w and q12w SC regimens, except in patients with high inflammatory burden, where q8w ustekinumab was favourable. In the extension study, no new safety signals were identified between Week 96 and Week 156, rates of adverse events and serious adverse events were comparable to placebo through Week 156, and antibody formation remained low.
Clinical Insights in Inflammatory Bowel Disease
Doctor Tim Raine
Addressing questions around why remission rates among patients on placebo were high in IM-UNITI, Dr Raine explained that subjects were only enrolled in the study after responding to ustekinumab IV induction. Ustekinumab has a long half-life, with the consequence being that many placebo patients would continue to have the drug in their systems.
The first case was a 21-year-old female non-smoker diagnosed with Crohn’s disease in 2014 after 14 months with abdominal pain. The patient had L3 (ileocolonic) involvement and B1 (non-stricturing, non-penetrating) disease, Harvey Bradshaw Index (HBI) was 10, weight 55 kg, CRP 30 mg/dL, haemoglobin (Hb) 121 g/dL, albumin 33g/L, and fCal 1,100 µg/g. The patient, who had been prescribed one previous course of corticosteroids, was initiated on azathioprine 2.5 mg/kg. By 2015, her symptoms had deteriorated, and she was experiencing abdominal pain and diarrhoea with intermittent bleeding; HBI was 14 and colonoscopy revealed pancolonic skip lesions, ulceration, and bleeding. Addition of infliximab (5 mg/kg) to azathioprine produced clinical and biological remission (HBI was 2, CRP 4 mg/dL, and fCal 75 µg/g). However, by 2016 she had lost response (HBI was 11, infliximab trough level 1.1 µg/mL, thioguanine nucleotide 312 [normal], CRP 20 mg/dL, fCal 300 µg/g, and no antibodies to infliximab). At this point, infliximab was escalated to 10 mg/kg q8w. In 2017, the patient continued to have mild-to-moderate abdominal pain and diarrhoea with some bleeding; HBI was 10, CRP 9 mg/dL, Hb 121 g/ dL, albumin 33 g/L, and fCal 1024 µg/g. Endoscopy revealed ileocolitis with large superficial ulcerations (>2 cm) and a few deep ulcerations and MR-enterography terminal ileum wall thickening. The patient was Clostridium difficile negative.
An audience poll found that more than half (54.9%) of respondents would always measure drug levels and antidrug antibodies at this stage, 13.5% would do so sometimes, 17.3% were not convinced it was useful, and 14.3% did not have access to assays.
Commenting on the vote, Prof Armuzzi said his centre did measure drug and antibody levels in patients losing response. Prof Ghosh said that in the case of this patient there was sufficient evidence to switch to a biologic targeting a different pathway; he added that it was important to get access to the assays to make a rational decision.
The patient had infliximab trough levels of 7 µg/mL and no antidrug antibodies but was experiencing joint manifestations. Considering the next step, 70.8% of the audience voted to switch from infliximab to ustekinumab, 13.8% to switch from infliximab to adalimumab, 10.8% to switch from infliximab to vedolizumab, 4.1% to continue infliximab and switch azathioprine to methotrexate, and 0.5% for ‘other.’
The patient was recommended biologic treatment with an alternative mode of action to anti-TNF, and, based on joint manifestations and the need for rapid onset of action, was given ustekinumab IV induction.
Regarding combining azathioprine and ustekinumab, 55.4% of the audience voted to stop azathioprine immediately, 32.8% to continue azathioprine for 6–12 months and then stop, 7.7% to switch from azathioprine to methotrexate immediately; and 4.1% for ‘something else.’
Prof Armuzzi suggested that combination therapy with azathioprine might provide benefits in the first few months of biologic treatment by reducing drug immunogenicity. Prof Ghosh added that he would discontinue azathioprine 6–12 months after starting ustekinumab, but only used azathioprine in patients already taking it.
Data from IM-UNITI shows that median ustekinumab serum concentrations over 44 weeks are unaffected by concomitant immunomodulators (including azathioprine, 6-mercaptopurine, or methotrexate).4
The patient was started on weight-tiered (˜6 mg/kg) ustekinumab IV and azathioprine was continued. At Week 8 the patient showed clinical improvement, but not a complete response (HBI fell from 10 to 7), and received ustekinumab 90 mg SC.
Reviewing response prior to Week 16 following Week 8 maintenance ensures enough time for an additional Week 16 dosing if response is inadequate. At Week 16 clinicians have three choices:6
- For patients who show an adequate response, no additional ustekinumab dose is required (they can be dosed instead at Week 20).
- For patients who show an inadequate response, ustekinumab 90 mg SC is given at Week 16.
- For patients who show no benefit, treatment is discontinued at Week 16 (with patients considered for different treatments).
Key ustekinumab dosing decisions are made just prior to Week 16, rather than Week 8, the time of the first maintenance SC dose.1,6 The reason is that 50% of patients who do not respond to IV loading achieve clinical responses after the first SC dose.1,6
Responding to the question of how to assess ustekinumab response prior to Week 16, 56.5% of the audience voted to use biomarkers (CRP, fCal), 17.5% to use patient symptoms, 12.3% to use colonoscopy; 10.4% to use HBI; 1.9% to use small bowel ultrasound; and 1.3% to use ‘other methods’.
Prof Ghosh commented that when considering dose escalation, biomarkers should be assessed with clinical information between Week 15 and a day or so before Week 16 to avoid delaying dose escalation.
At Week 16 the patient received ustekinumab 90 mg SC and achieved clinical remission (HBI 4, CRP 5 mg/dL, fCal 162 µg/g). Azathioprine was stopped.
In IM-UNITI, clinical remission at Week 44 was similar for the different ustekinumab dosing schedules: 48.8% for ustekinumab 90 mg q12w SC versus 53.1% for ustekinumab 90 mg q8w SC.1 However, in a post-hoc UNITI analysis, patients with high baseline inflammatory burden (CRP ≥5 mg/L) achieved greater benefits from q8w dosing.3 Furthermore, an endoscopic sub-study involving three Phase III studies determining the safety and efficacy of ustekinumab (IV induction and SC maintenance) showed that patients were more likely to achieve endoscopic endpoints with q8w than q12w ustekinumab maintenance.7
In the second case study, delegates considered a 37-year-old female business consultant, non-smoker, diagnosed with Crohn’s disease in October 2016. The patient experienced moderate-to-severe abdominal pain, increased stool frequency, and weight loss. Colonoscopy revealed ileocaecal and transverse colon involvement (including 25 cm of the ileum). Other available data included weight 58 kg, HBI 8, CRP 30 mg/dL, and fCal 850 µg/g. The patient took steroids at diagnosis and had azathioprine initiated (2.5 mg/kg).
The patient showed clinical improvement (HBI 6) and, in January 2017, had steroids discontinued, but continued to experience intermittent low-grade abdominal pain and diarrhoea. In April 2017, colonoscopy revealed aphthous ulcers in the ileum and transverse colon, but no fistulisation. Other results were HBI 11, CRP 15 mg/dL, fCal 350 µg/g, and 6-thioguanine 260 pmol/8×108 red blood cells. Additionally, she reported frequent coughs, colds, and recurrent herpes simplex.
Considering the next steps, 39.9% of the audience voted to start anti-TNF therapy, 33.9% to start ustekinumab, 17.3% to start vedolizumab, 7.1% for a course of steroids and to continue azathioprine, and 1.8% for ‘other’ treatments.
The UNITI-2 study demonstrated ustekinumab to be a potential treatment option for patients naïve to biological treatment, with 40.2% of patients receiving ustekinumab achieving clinical remission at Week 8 (Crohn’s Disease Activity Index score <150).1 Additionally, and of relevance to patients experiencing recurrent infections, ustekinumab showed similar infection-rate data to placebo.9 In IM-UNITI, 49.6% of patients taking placebo had infections versus 46.2% on ustekinumab 90 mg q12w SC versus 48.1% on ustekinumab 90 mg q8w SC.1 Additionally, the Psoriasis Longitudinal Assessment and Registry (PSOLAR) demonstrated cumulative incidence infection rates per 100 patient-years of 0.83 for ustekinumab versus 2.49 for infliximab versus 1.97 for adalimumab.8
For this patient, ustekinumab at 390 mg IV (based on the weight-tiered dose regimen of ˜6 mg/kg) was started based on the infection-rate data,1,9 onset of action,1,10 no requirement for immunomodulators,4 and low incidence of anti-drug antibodies.1 Azathioprine was discontinued.
By Week 8, the patient demonstrated a clinical response (HBI 8) and received ustekinumab 90 mg SC. At Week 14 (prior to a potential Week 16 dose) she reported feeling well, with reduced pain and stool frequency (HBI 5, CRP 12 mg/ dL, and fCal 180 µg/g). However, clinical remission had not been achieved and she received ustekinumab 90 mg SC at Week 16 (q8w dosing). From then on, the patient entered remission (HBI 3 at Week 24) and remained in remission with HBI 0 at Week 32, HBI 2 at Week 40, and HBI 0 at Week 48.
Commenting, Prof Ghosh said he would choose a biological anti-TNF first-line treatment with reduced risk of exacerbating infections for patients with upper respiratory tract and herpes simplex infections. Prof Armuzzi added that he would have selected ustekinumab for the current patient based on its safety profile and onset of action. Based on a post-hoc analysis, he stated that ustekinumab’s onset of action could take a few days.10
Future Options in Inflammatory Bowel Disease
Professor Alessandro Armuzzi
IBD is a growing condition, with over 3 million people worldwide affected by UC.11
A Danish population cohort demonstrated UC to be progressive, with 35% of patients with proctitis or left-sided colitis at diagnosis progressing to extensive colitis in the 10 years following diagnosis.12 Furthermore, patients whose condition extended to proctitis or left-sided colitis were at greater risk of experiencing colectomy at 10 years than those who had not extended.12
A second population cohort showed that, at 9 years post diagnosis, the cumulative probability of surgery in UC patients had fallen, decreasing from 14.5% for the period 1979–1986 to 9.1% for the period 2003–2011 (p<0.001).13 However, between 1995 and 2011, the cumulative probability of colectomy was higher for patients prescribed corticosteroids and/or azathioprine than for those not prescribed these medications.13
The French BIRD PRO study analysis of 1,185 patients demonstrated UC to have extensive personal impact beyond physical impairment.14 The study, using 6 self-reported questionnaires, showed 53.3% of subjects reported poor quality of life, 47.4% severe fatigue, 49.4% depression, and 30.3% anxiety.14 Furthermore, about half of the subjects reported presenteeism (being at work for more hours than required), moderate-to-severe loss of work productivity, and loss of activity.14
A 2016 Italian consensus treatment algorithm for moderate-to-severe UC shows that patients should be started with glucocorticosteroids and aminosalicylate (5-ASA).15 If found to be steroid refractory, at this point they would be switched to biologics/small molecules; if not steroid refractory, glucocorticosteroids would be tapered and 5-ASA and azathioprine continued.15 If found to be steroid dependent, intolerant, or refractory to 5-ASA/azathioprine, patients would be switched to biologics/small molecules (Figure 2).15
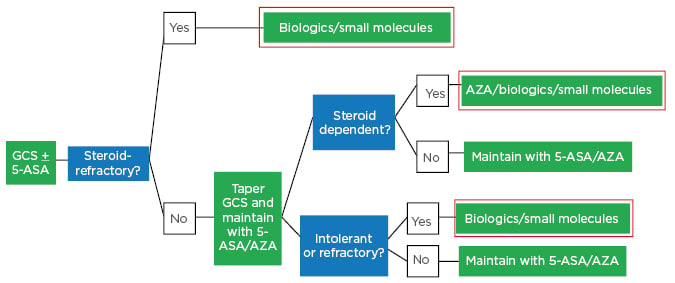
Figure 2: Italian ulcerative colitis treatment algorithm in 2019: Moderate-to-severe ulcerative colitis.
5-ASA: aminosalicylate; GCS: glucocorticosteroids.
Adapted from Armuzzi A et al.15
The goals of UC therapy are to achieve clinical and endoscopic remission as soon as possible, maintain remission without steroids, and ultimately achieve histological remission.15 There is a need to prevent complications, optimise time of surgery, and improve quality of life.15
When conventional therapy does not work in moderate-to-severe UC, four different biologics (infliximab, adalimumab, golimumab, and vedolizumab) and one small molecule (tofacitinib) are available.
Many pathways have been studied in the intestinal mucosa for UC treatment;16-18 treatments targeting these pathways include inhibitors of leucocyte trafficking (ozanimod and etrasimod), molecules inhibiting cytokine signalling through blocking Janus kinases (e.g., tofacitinib, filgotinib, and upadacitinib), and molecules directed at IL-23 and IL-12 (ustekinumab, guselkumab, risankizumab, mirikizumab, and brazikumab).16-18
In the Phase III UNIFI study, 961 patients with moderate-to-severe UC were randomised 1:1:1 to receive induction placebo (n=319), ustekinumab 130 mg IV (n=320), or a weight-tiered ustekinumab IV dose (˜6 mg/kg [n=322]).19 Participants achieving clinical response at the end of induction (Week 8) were eligible for the maintenance part of the study.19
Results at Week 8 showed that clinical remission (primary endpoint defined as Mayo score ≤2 points with no individual sub-score >1) was achieved in 5.3% of patients taking placebo, 15.6% taking ustekinumab 130 mg IV (p<0.001), and 15.5 % taking ustekinumab at ˜6 mg/kg (p<0.001).19 When the biological-failure subgroup was examined, it was found that clinical remission was achieved in 1.2% of patients taking placebo, 11.6% taking ustekinumab 130 mg IV (p<0.001), and 12.7% taking ustekinumab at ˜6 mg/kg (p<0.001). Further analysis of change from baseline in partial Mayo scores and fCal demonstrated onset of action with marked differences occurring between placebo and ustekinumab arms around Week 2.19
Exploring secondary endpoints at Week 8:19
- Endoscopic healing occurred in 13.8% of patients taking placebo, 26.3% taking ustekinumab 130 mg IV (p<0.0001), and 27% taking ustekinumab IV at ˜6 mg/kg (p<0.001).
- Clinical response occurred in 31.3% of patients taking placebo, 51.3% taking ustekinumab 130 mg IV (p<0.001), and 61.8% taking ustekinumab IV at ˜6 mg/kg (p<0.001).
- Mucosal healing (endoscopic and histological healing) occurred in 8.9% of patients taking placebo, 20.3% taking ustekinumab 130 mg IV (p<0.001), and 18.4% taking ustekinumab IV at ˜6 mg/kg (p<0.001).
- Change from baseline in IBD questionnaire occurred in 10% of patients taking placebo, 31.5% taking ustekinumab 130 mg IV (p<0.001), and 31% taking ustekinumab IV at ˜6 mg/kg (p<0.001).
- Key safety data showed no differences between placebo and ustekinumab for adverse events, serious adverse events, infection and serious infections, and adverse events during 1 hour of infusion.
New data presented at ECCO 2019 takes the UNIFI study story of UC patients treated with ustekinumab forward to Week 44 (52 weeks after IV ustekinumab induction).20 First, an abstract by Dr Bruce Sands showed that the primary endpoint of clinical remission at Week 44 occurred in 24.0% of patients receiving placebo, 38.4% receiving ustekinumab 90 mg q12w SC, and 43.8% receiving ustekinumab 90 mg q8w SC.20 It was noteworthy that, at Week 44, ustekinumab continued to be effective in biologic-failure patients, with clinical remission for this group occurring in 22.7% of patients receiving placebo versus 25.7% receiving ustekinumab 90 mg q12w SC and 45.1% receiving ustekinumab 90 mg q8w SC.20
An oral presentation by Dr Gert van Assche presented at ECCO 2019 exploring secondary UNIFI endpoints found that, at Week 44:21
- Durable partial Mayo remission (defined as achievement of ≥80% of all visits prior to Week 44 and at Week 44) was achieved in 35.4% of patients taking placebo, 48.3% of patients taking ustekinumab 90 mg q12w SC (p=0.010 versus placebo), and 57.4% of patients taking ustekinumab 90 mg q8w SC (p<0.01 versus placebo).
- Symptomatic remission (defined as achievement of ≥80% of all visits prior to Week 44 and at Week 44) was achieved in 45.9% of patients taking placebo, 63.1% taking ustekinumab 90 mg q12w SC (p=0.009 versus placebo), and 66.4% taking ustekinumab q8w (p=0.002 versus placebo).
- Endoscopic improvement was achieved in 35.2% of patients taking placebo, 60.3% of patients taking ustekinumab 90 mg q 12w SC (p=0.002 versus placebo), and 64.9% of taking ustekinumab q8w (p<0.001 versus placebo).
- Inflammatory Bowel Disease Questionnaire (IBDQ) remission (defined as IBDQ remission score ≥170) was achieved in 49.5% of patients taking placebo, 68.8% of patients taking ustekinumab 90 mg q12w SC (p=0.002 versus placebo), and 66.0% patients taking ustekinumab q8w (p=0.019 versus placebo).
A third abstract presented at ECCO 2019 revealed ustekinumab to have beneficial effects on patient quality of life.22 The study, assessing the general health status of UC patients from UNIFI, showed that the proportion of patients with clinically meaningful improvements in the Short Form Health Survey (SF-36) physical and mental component summaries (≥5 points) and EuroQoL-5D Health Questionnaire (EQ-VAS) (>10 points) at Week 44 was significantly greater for both ustekinumab groups compared to placebo (p≤0.001 for all).22 Notably, significant differences for SF-36 physical component summary, SF-36 mental component summary, and EQ-visual analogue scale scores were found between both ustekinumab groups and placebo as early as Week 8 (p<0.001 for all), demonstrating the effect of ustekinumab on quality of life.22
In conclusion, for moderate-to-severe UC, a single IV ustekinumab infusion (130 mg or ˜6 mg/kg) induced clinical remission, clinical response, endoscopic healing, mucosal healing, improved health-related quality of life, and had an adverse event profile consistent with known safety profiles at Week 8.








