Meeting Summary
The objectives of this symposium were to describe the current unmet needs in the treatment and management of inflammatory bowel diseases (IBDs) in clinical practice. Crohn’s disease (CD) is a chronic inflammatory disease affecting several areas of the gastrointestinal tract, which can have a negative impact on patient quality of life (QoL) and may lead to disability. Effective management and early disease intervention combined with control of inflammation in CD are crucial to achieving sustained remission. Clinical remission, however, is not always an indicator of mucosal healing and does not necessarily translate to real-world benefits for patients. Unfortunately, not all patients respond to their current treatment and several experience unacceptable adverse events. Furthermore, treatment with some anti-tumour necrosis factor (TNF) antibodies can paradoxically induce psoriatic lesions that regress after treatment withdrawal, highlighting the need for more therapeutic options. The symposium was opened by Prof Séverine Vermeire, who discussed the unmet needs for patients with IBD and whether CD is sufficiently controlled. Special attention was paid to clinical remission, steroid-free remission, and mucosal healing. Dr Alessandro Armuzzi then reflected upon the current therapeutic options for CD and their application in clinical practice. The final contribution came from Prof Laurent Peyrin-Biroulet, who discussed new developments in the treatment of IBD, and presented data from clinical trials of the monoclonal antibody (mAb) ustekinumab.
Unmet Medical Needs: Is Crohn’s Disease Sufficiently Controlled?
Professor Séverine Vermeire
When discussing CD, the focus is generally placed on clinical remission, steroid-free remission, and mucosal healing, which are the endpoints used to reflect efficacy during the development of new therapeutic compounds. While the situation is improving, there is still a long way to go. Data from the randomised SONIC trial demonstrated that patients treated with the anti-TNF biologic infliximab plus the immunosuppressant azathioprine or infliximab alone were statistically significantly more likely to achieve steroid-free remission (at Week 26) than with azathioprine alone, but that the most efficacious option was the combination regimen (57% of patients) versus 44% with infliximab (p=0.02) and 30% with azathioprine monotherapy (p<0.001).1 A similar scenario was observed for mucosal healing (at Week 26), rates of which were higher with the combination therapy (44%) than with monotherapies (30% [p=0.06] and 17% [p<0.001], respectively).1 However, for both endpoints, 43–56% of patients did not achieve therapeutic success. There has been an increase in the rate of surgical resections for CD over the past few decades.2,3 For a recent cohort from Scandinavia, to whom biologic agents were available, there was a reduction in the number of surgical interventions.3 Nonetheless, the rates are still increasing and are >20% after 5 years since diagnosis of the disease (Figure 1). There remains a clear need for broad surgical resections in some patients. Furthermore, several factors that are either not considered or are discussed only briefly, including QoL, fatigue, depression, anxiety, sleep disturbance, arthralgias, other extra-intestinal manifestations, and the side effects of drugs, can lead to patient dissatisfaction. Progress is required to prevent cumulative bowel damage and ultimately surgical resection.
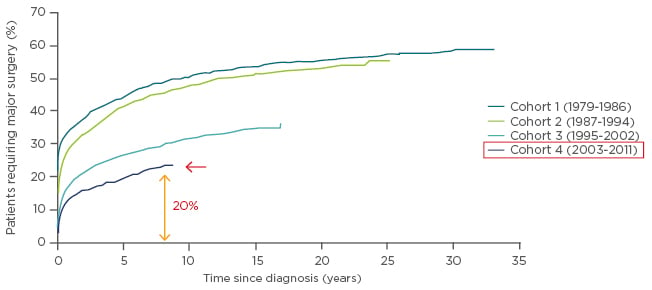
Figure 1: Cumulative probability of first major surgery among 13,185 patients with Crohn’s disease relative to time since diagnosis and according to cohort at diagnosis.
Adapted with permission from Rungoe et al. 2014.3
Current Therapeutic Options in the Treatment of Crohn’s Disease: Where Do We Stand?
Doctor Alessandro Armuzzi
The goals of therapy are to induce remission both clinically and endoscopically, maintain steroid-free remission, prevent complications, optimise the timing of surgery, and improve the patient’s QoL. For patients with active CD, it is common to start with systemic steroids (Figure 2), with the next step in the therapeutic journey depending upon the patient’s response to treatment.4 Regardless of the choice of therapy, the treatment of CD remains problematic in around half of patients, and many develop complications and ultimately need to undergo surgery, sometimes more than once (following relapses).5
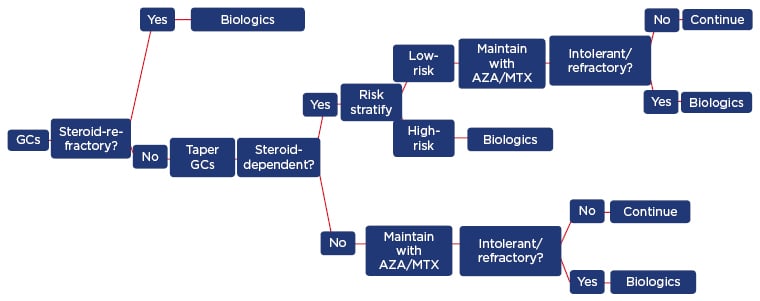
Figure 2: Crohn’s disease treatment algorithm in clinical practice.
AZA: azathioprine; GCs: glucocorticosteroids; MTX: methotrexate.
Adapted with permission from Armuzzi et al. 2016.4
One option towards improving the situation for patients with CD is to change the therapeutic strategy. In some cases the traditional strategy is the best one, with ‘step-up care’, involving sequential and incremental prescription of corticosteroids and immunosuppressants before going on to biologics upon treatment failure/relapse. However, this strategy does not prevent disease progression and is associated with an increased risk of side effects due to repeated use of corticosteroids.6 Some of these long-term complications can be avoided by implementing an accelerated step-up or an early top-down approach, in which the treatment starts with combination immunosuppression with corticosteroids, missing out the steroid-alone step, or starting with immunosuppression plus biologic therapy.6 In both these strategies, the early introduction of biologics may expedite mucosal healing, thus reducing the likelihood of surgery. However, data supporting the best choice of approach are lacking, thus physicians must currently rely on their personal experience, and perhaps clinical predictors or a lesion identified by endoscopy or radiology to direct the treatment decision-making process.
Data from observational studies suggest that corticosteroids are the best choice for resolving symptoms in >80% of patients; however, around 15% are steroid refractory and by 1 year, one-third of patients have become steroid-dependent and have become at risk for chronic disease, steroid-induced side effects, and surgery.7-9 Therefore, while steroids are good for symptom control, they are poor predictors of outcome in most patients with CD worldwide.
Until recently, the use of thiopurines was an alternative to steroids for treating CD and was supported by studies of large cohorts of patients demonstrating long-term benefits. However, doubt has been cast over their effectiveness in this patient population, with studies demonstrating a lack of superiority over either conventional management10 or placebo11 with respect to time to clinical relapse and achievement of corticosteroid-free remission, respectively. Furthermore, thiopurines are associated with a significant risk of lymphoma in patients with IBD with only 1 year of exposure, and particularly among those aged >50 years.12 Therefore, thiopurines may only provide some benefit in a subpopulation of patients with CD and, like steroids, prolonged use could potentially be dangerous.
Newer options include biologics, which have been under scrutiny for CD since early 2000, when it was established that anti-TNF agents were efficacious after the failure of traditional therapies. The benefits of biologics include clinical response/remission, steroid-free remission, mucosal healing, fistula healing, reduced rates of hospitalisation and surgery, and improved QoL.1,13-29 Anti-TNFs have been used variously for IBD over the past 15 years as a monotherapy, as part of combination therapies, in treatment-naïve patients, and following postoperative recurrence. Clinical trials with the anti-TNF mAbs infliximab and adalimumab in CD have demonstrated sustained clinical benefit in 63% and 71% of patients, respectively, but with dose escalation being required in 50% and 34%, respectively.30,31 In addition, ≤10–20% of patients are non-responders initially, and 15–20% of patients per year become treatment refractory.30,31 The risk of opportunistic infection, which is doubled with the use of anti-TNF therapy, must also be borne in mind during the treatment decision-making process.32
In summary, while it is possible to manage CD using currently available drugs and regimens in many patients, including swapping between and within drug classes,15,20,21,30,33-41 the success rate is not 100% and additional strategies are required to prevent mucosal damage and surgery.
New Developments in the Treatment of Inflammatory Bowel Diseases
Professor Laurent Peyrin-Biroulet
When patients become refractory to treatment or suffer unacceptable toxicities, it becomes necessary to swap to either another agent within the same class or to switch classes (Figure 2). Ustekinumab, a fully human immunoglobulin G1κ mAb has a novel mechanism of action compared with other biologics; it inhibits signalling mediated by interleukin-12 and interleukin-23, key pro-inflammatory cytokines in the pathogenic immune cascade of CD. Ustekinumab is currently indicated for the treatment of plaque psoriasis (adult and paediatric) and psoriatic arthritis (alone or in combination with methotrexate),42 and has been tested as an induction therapy in patients with CD who have failed on anti-TNF therapy (UNITI-1) or conventional therapy (UNITI-2), and as a maintenance therapy in responders from those two trials (IM-UNITI) (Figure 3).43
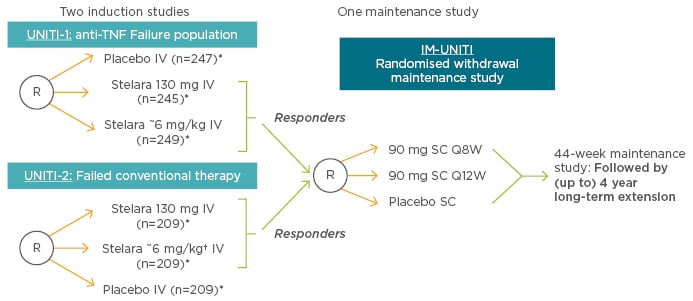
Figure 3: Phase III UNITI trials for ustekinumab in Crohn’s disease.
*Subjects randomised to placebo and subjects who are non-responders to Stelara are eligible for non-randomised maintenance dosing after completion of the induction study.
†Ustekinumab is not indicated for the treatment of inflammatory bowel disease.
IV: intravenous; Q8/12W: every 8/12 weeks; R: randomised; SC: subcutaneous; TNF: tumour necrosis factor.
Adapted with permission from Rutgeerts et al. 2016.47
Induction efficacy was demonstrated in both anti-TNF and conventional therapy-refractory patients. In UNITI-1, clinical response at Week 6 was observed in 34% of patients receiving intravenous (IV) ustekinumab 130 mg and 6 mg/kg, and in 22% of those on placebo. In UNITI-2, these figures were 52%, 56%, and 29%, respectively.43 Ustekinumab thus benefits 50–60% of patients, and is more effective than placebo (difference of >10%). The time to reach peak efficacy of anti-TNFs is 2–3 months; however, with ustekinumab these clinical response data need to be validated.
To achieve full control of CD, the focus must be disease remission and symptom control that enable the patient to return to a normal life. For patients who were anti-TNF-refractory, rates of clinical remission at Week 8 were 16%, 21%, and 7% for those receiving ustekinumab 130 mg and 6 mg/kg, and placebo, respectively; for patients who were conventional therapy failures, those figures were 31%, 40%, and 20%, respectively.43 These findings suggest that the preferred dose should be 6 mg/kg. The documented pharmacokinetic profile of ustekinumab in part explains the results obtained with the 6 mg/kg dose over the 130 mg flat dose in UNITI-1 and UNITI-2. There was a greater serum concentration of ustekinumab throughout the first 8-week period with the 6 mg/kg dose relative to the 130 mg flat dose. There was no difference in the pharmacokinetic profile between patients with and without a history of anti-TNF therapy. Moreover, ustekinumab appears to be minimally immunogenic, with an overall incidence of antibodies at the final safety visit of 0.2%.44
In the maintenance trial, there was a subgroup of patients who were not in clinical response to ustekinumab induction. These patients received a subcutaneous injection of ustekinumab at Week 0 on entry into the maintenance study, and were assessed for response at Week 8. In the patients who were non-responders to the 130 mg or 6 mg/kg induction schedules and received an injection of 90 mg ustekinumab at Week 0 of IM-UNITI, 51% of patients achieved clinical response, and 29% achieved clinical remission by Week 8, which is 16 weeks after the initial induction regimen. Despite non-response at induction with intravenous ustekinumab, there is still the possibility of response with a subcutaneous regimen.45
Of the responders from the UNITI-1 and 2 trials who entered IM-UNITI, 49% and 53% of patients taking subcutaneous (SC) ustekinumab 90 mg every 8 weeks (Q8W) and every 12 weeks (Q12W), respectively, demonstrated clinical remission as determined by a Crohn’s Disease Activity Index (CDAI) score of <150 at Week 44 (primary endpoint). Although 36% of those taking placebo also had a response, these patients had already responded to IV ustekinumab in the UNITI-1 and 2 trials and therefore already had good disease control. There was an additional 18% difference between the ustekinumab and placebo arms. Furthermore, the proportion of patients achieving steroid-free remission at Week 44 was higher with SC ustekinumab Q12W and Q8W (43% and 47%, respectively) than for placebo (30%).46 Objective (i.e. biomarker) signs of clinical remission (change in serum C-reactive protein [CRP] from baseline, faecal calprotectin, and a ≥3-point reduction in baseline Simple Endoscopic Score for Crohn’s Disease [SES-CD] score at Week 8 of induction) also demonstrated the superiority of ustekinumab over placebo.46
Flexibility of dosing is important as some patients may not achieve a response or remission, but it can be addressed towards improving their outcome perhaps by altering the frequency of injections from 12 to 8 weeks, for example. However, patients differ in inflammatory burden, and altering the frequency of injections may harm a patient,45 while in others it may be successful. Further research is required to clarify this situation to establish the subpopulations for whom dose adjustment would be beneficial.
Ustekinumab is known to have a good safety profile in other patient populations, and preliminary findings indicate that it may be a suitable treatment option in patients with CD, although confirmation of that finding is required. In summary, ustekinumab has demonstrated promising clinical results for both induction and maintenance therapy in patients with CD with a variety of therapeutic histories, and is minimally immunogenic. This novel mAb has the potential to become the next step towards the goal of maintained clinical remission in all patients with CD.
Question and Answer Session
Q: What are important factors that we need to take into account during the treatment decision-making process, and to what extent do we need to involve the patient?
Dr Armuzzi: The patient should always be involved. The data to date show that ustekinumab can work for both biologic-naïve and refractory cases, and so could be suitable for whichever condition is encountered in the clinic. It is important to consider speed of effect, and choose an agent that acts rapidly, particularly in patients with an extra-intestinal manifestation that is not the only condition.
Prof Vermeire: Speed of effect is important because the goal is to make sick patients well, and while it has been demonstrated that ustekinumab will do that, anti-TNFs are also effective. It is also important to treat the patient as a whole, including extra-intestinal manifestations. There is flexibility of dosing with anti-TNFs, and there are already data on how to measure and optimise their usage. The data on flexibility and dosing of ustekinumab presented here were interesting in that regard. Perhaps therapeutic drug monitoring would be useful, or reducing the interval between injections.
Prof Peyrin-Biroulet: It may be an advantage to have a maintenance SC agent, particularly in young patients. In addition, the interval between injections is important from the patient’s perspective, as it potentially allows them to forget the disease but not the drug. Patients may feel that a high frequency of injections is a constant reminder of the disease. Therefore, it may be an advantage to inject every 3 months.
Prof Vermeire: Or even every 2 months.
Prof Peyrin-Biroulet: Another consideration is cost, especially since diagnosis will likely become inexpensive. Anti-TNFs are a good option clinically with a low cost. The cost of ustekinumab must be established. Safety is also an important factor. There are no comparative data between ustekinumab and other agents at this point. A large prospective cohort of patients is needed tocompare the effectiveness and safety of the various drugs, not all of which will be feasible for all patients. Flexibility is also clearly an advantage, and more data are required to determine this parameter with novel compounds.
Q: Are there any data yet as to whether there is any benefit of co-prescription of azathioprine?
Prof Vermeire: The question is whether ustekinumab should be used as a monotherapy or as part of a combination regimen.
Prof Peyrin-Biroulet: There are no data as yet, though it appears likely that there will be no benefit with azathioprine co-prescription. At this point, physicians must rely on what is currently known. Ustekinumab therapy is only minimally immunogenic, so in practice it could be used as a monotherapy. Combination therapy with azathioprine should only be implemented for safety reasons; it should be avoided where possible.
Dr Armuzzi: There are only preliminary data on this at present; but since the rate of immunogenicity is very low with ustekinumab, the potential for monotherapy is very attractive.
Q: Do the psoriasis data provide any information regarding situations in which anti-TNF therapy is contraindicated, such as patients with multiple sclerosis?
Prof Peyrin-Biroulet: Theoretically, in terms of the pathogenesis of CD, this should not be a contraindication for the use of ustekinumab. Expert knowledge is required to adequately answer this question.
Dr Armuzzi: According to the registry for psoriasis, there is no signal that this drug could increase the risk of developing demyelinating diseases.
Prof Vermeire: The question is whether it is possible to extrapolate the profile of psoriasis with CD, but these are two different diseases with different pathogenetic profiles.
Dr Armuzzi: As mentioned in the presentations, there are safety data for just 1 year of treatment with ustekinumab, which gives only a preliminary indication of safety in CD. The data in the dermatologic registry, with thousands of patients treated with ustekinumab, some of whom will have associated IBD, have raised no signals. While this is not a definitive answer, some assurance can be taken from the safety profile of this drug in those patients with psoriasis and IBD. The premise is that ustekinumab has a good safety profile in both the short and long-term.
Prof Peyrin-Biroulet: Discussions with dermatologists regarding the use of this drug, while hearsay and in no way definitive, allow us to conclude that the safety profile of ustekinumab is good.
Prof Vermeire: The data are certainly reassuring.
Q: Are the data on fistula available?
Prof Peyrin-Biroulet: There are no specific trials. However, data from the subgroup of patients with fistulising disease, which are not yet in the public domain, are quite promising. Perianal fistulae usually arise from rectal ulcers, so if mucosal healing occurs there will be less intestinal inflammation and so there is no reason why perianal fistula healing should not also be observed, although it may take longer.
Q: Why there is a focus on extra-intestinal manifestations rather than the primary disease, IBD?
Dr Armuzzi: The drug could cover the bowel as well as what is outside of the bowel; it is a systemic drug with what appears to be a promising safety profile, and rapidly clears systemic inflammation. In the first instance, the focus is on the patient with extra-intestinal manifestations, but there are many other situations in which the drug could be applied. Moreover, it is a primary biologic in some situations. It therefore has flexibility, not only in dosing, but in its application, with the added value of requiring only single injections. The next step is to establish the patient subpopulation most likely to achieve the best response from this agent.
Prof Peyrin-Biroulet: The data on CRP show a systemic effect. Extra-intestinal manifestations are driven by intestinal inflammation; in bad cases, it is important to heal the mucosa. In those cases, lowering the CRP should be the first task; ustekinumab may be the drug of choice here.
Q: Are combination biologics the way forward in the future towards improving remission rates?
Prof Peyrin-Biroulet: A key point is early disease control. Data yielded from studies on anti-TNF agents is that the rates of remission and mucosal healing can be significantly increased when patients with early CD are treated.
Dr Armuzzi: Biological predictors are awaited that could help in the therapy decision-making process.
Prof Peyrin-Biroulet: Combination therapies are already in use; anti-TNF agents are used in combination with steroids in many patients. It is documented that this combination does not improve the safety profile. However, comparative trials are required to establish the superiority of one mAb over another.








