Meeting Summary
The symposium, entitled “Burning questions in IBD: Learnings from emerging drug options and clinical cases,” took place during the 2019 United European Gastroenterology (UEG) Week annual congress in Barcelona, Spain. Distinguished experts Prof Peyrin-Biroulet, Prof Vermeire, and Prof Panés tackled several of the outstanding questions in inflammatory bowel disease (IBD) management, focussing the discussion on treat-to-target strategies and how these could be applied in IBD management; when to initiate biologic treatments, and the factors involved in making these treatment decisions; the use of ustekinumab in ulcerative colitis (UC) management; efficacy and safety of biologics; and whether monotherapy or combined treatment is the optimal treatment approach in IBD. The experts used informative patient cases and data from current clinical studies to help illustrate the possible solutions to each ‘burning question’, incorporating questions from the audience into each discussion.
What Should be the Treatment Target in Crohn’s Disease and Ulcerative Colitis, and How Should the Treat-To-Target Strategy be Applied for Both Diseases?
Professor Laurent Peyrin-Biroulet
The treatment armamentarium is expanding for patients with UC and Crohn’s disease (CD). The treatments currently available include anti-inflammatory, immunosuppressant, and corticosteroid therapies, as well as small-molecule treatments and several biologic treatments targeting TNFα, α4β7 integrins, and IL-23/12.1,2
Treatment targets can include factors such as improved quality of life, and clinical, biochemical, and endoscopic remission. Though perhaps not every goal can be achieved in an individual patient, applying a treat-to-target strategy could be useful for patients. Treat-to-target can be defined as “a treatment strategy in which the clinician treats the patient aggressively enough to reach and maintain explicitly specified and sequentially measured goals.”3 Treat-to-target is especially attractive in IBD management, as this strategy is proactive and offers a clear goal for patients.3,4
There is a current need for personalised and ‘top-down’ treatment in UC and CD; this is not solely to treat the symptoms, but to alter the disease course and improve disease burden to the point at which the patient can once again lead a normal life.5 For patients with CD, the 2015 Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) recommendations state treatment targets that include resolution of abdominal pain, normalisation of bowel habits, and an absence of ulcerations within an approximated 6-month period. For patients with UC, treatment targets include resolution of rectal bleeding, normalisation of bowel habits, and a Mayo endoscopic subscore of 0 (0 is considered optimal, a subscore of 1 should be considered the minimum) within a 6-month period.4 Patients should be closely monitored during the initial period, with possible ‘step-up’ of treatment dosing being implemented if necessary (Figure 1).
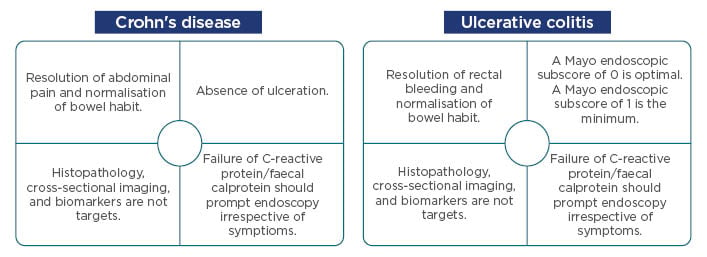
Figure 1: Current Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) recommendations for Crohn’s disease and ulcerative colitis.
Adapted from Peyrin-Biroulet et al.4
Currently, two clinical trials have examined the effect of ‘tight monitoring’ and treat-to-target strategies (CALM and STARDUST, respectively) on clinical outcomes in patients with CD.6,7 The design of the CALM study aimed to examine the use of a tight monitoring algorithm on treatment outcomes in patients with CD, using biomarkers such as faecal calprotectin, C-reactive protein, and clinical symptoms as drivers for dose escalation or de-escalation during the 48-week trial period.6 The landmark STARDUST treat-to-target study consisted of an induction phase, a maintenance phase, and a long-term extension, with a primary outcome of endoscopic response as the treatment target at Week 48, with endoscopies performed at baseline, Week 16 (treat-to-target arm only), and Week 48.7
Remission may not be achievable in every patient, according to Prof Panés. He noted that mild-to-moderate symptoms may persist even if there is complete endoscopic healing, and mild symptoms may be an indicator of the need to proactively intensify treatment.
Treat-to-target is beginning to be adopted in clinical practice, according to Prof Peyrin-Biroulet, although proper patient education is an important factor for acceptance of this strategy.
When is the Appropriate Time to Start Treatment, either with a Biologic or Small-Molecule Therapy, and What Factors Should be Considered when Making Treatment Decisions?
Professor Laurent Peyrin-Biroulet
Appropriate timing of biologic treatment initiation often represents a challenge for clinicians, because there are no specific recommendations for the initiation of biologic treatment for patients with IBD.
Moreover, current European Crohn’s and Colitis Organisation (ECCO) guidelines do not offer guidance on choosing a biologic based on individual patient need, and delays in the representation of new treatments in the guidelines presents a further challenge when making treatment choices.
The results of the UNIFI study to evaluate the safety and efficacy of ustekinumab, a human monoclonal antibody against the p40 subunit of IL-23/12, showed that the primary endpoint of clinical remission at Week 8 of treatment versus placebo was met.8 Of all randomised patients in the study, approximately 62% showed a clinical response with the single intravenous induction dose of ustekinumab.8
Ustekinumab treatment also resulted in rapid and high efficacy in patients who did not respond to the induction dose, but who received a subcutaneous dose of ustekinumab dose at Week 8 and reassessment at Week 16; a combined analysis of data from Weeks 8 and 16 showed that approximately 78% of all patients showed a clinical response with ustekinumab by following the recommended dose schedule.9 Clinical response to ustekinumab was also observed in patients in whom disease had remained active on other biologic therapies.8
One of the most important treatment goals for patients with IBD is the achievement of steroid-free remission. The results from the UNIFI maintenance study indicate that, of those patients who were in remission at 1 year of treatment with ustekinumab, 97% were steroid-free (Figure 2).8
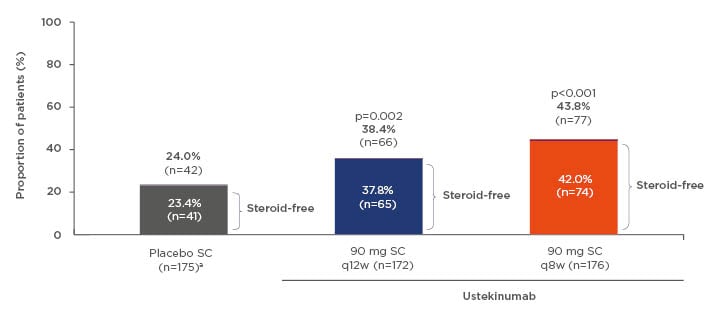
Figure 2: Patients in remissionb at Week 44c of maintenance therapy. aThe maintenance placebo population includes patients who received and responded to intravenous ustekinumab induction before receiving subcutaneous placebo. bMayo score ≤2; no individual subscore >1. cWeek 44 in maintenance is 1 full year of ustekinumab treatment (8-week induction + 44-week maintenance = 52 weeks in total).
q8w/q12w: every 8/12 weeks; SC: subcutaneous.
Adapted from Sands et al.8
Importantly, the UNIFI study was the first to use histoendoscopic mucosal healing as an endpoint. This endpoint includes both endoscopic improvement (endoscopy end score of 0 or 1) and histological improvement (0–5% neutrophils in the epithelium, no crypt destruction, and no erosions, ulceration, or granulation tissue).10 Patients receiving ustekinumab also showed endoscopic improvement at Weeks 8 and 44; significantly more patients treated with ustekinumab experienced histoendoscopic mucosal healing through 1 year versus placebo.8 Patients also reported improvements in Inflammatory Bowel Disease Questionnaire (IBDQ) scores through 1 year of treatment with ustekinumab versus placebo.11
In conclusion, the UNIFI trial data showed that induction and maintenance therapy with ustekinumab is associated with steroid-free remission, endoscopic improvement, histoendoscopic mucosal healing, improved quality of life, and early symptomatic improvement in patients with UC.8,12
Focus on Emerging Treatment Options in Ulcerative Colitis: How Should Ustekinumab be Used in the Treatment of Ulcerative Colitis?
Professor Séverine Vermeire
Ustekinumab is indicated for the treatment of adult patients with moderately to severely active UC who have had an inadequate or lost response, were intolerant to either conventional therapy or a biologic, or who have medical contraindications to such therapies.13 Prof Vermeire illustrated how ustekinumab treatment was used in the case of a male patient with UC and psoriasis who also developed synovitis.
The patient was initially treated with corticosteroids and continued to receive these during flares; he was eventually started on golimumab therapy and was later switched to infliximab in combination with azathioprine. Re-evaluation of the patient revealed no improvement; he was therefore enrolled in the UNIFI study and began receiving ustekinumab. The patient’s ustekinumab dose was escalated to every 8 weeks 2 years into the study; his faecal calprotectin levels began decreasing when he started showing clinical improvement and continued to decrease after a short stressful period when these levels temporarily increased. At this time, the patient has been receiving ustekinumab for almost 4 years, and is considered to be in clinical, biochemical, and endoscopic remission.
The faculty also discussed how optimising the dose of the current treatment can sometimes be a more effective strategy than switching to a different therapy. They also concluded that early treatment of inflammation with appropriate dosing is a necessary factor for treat-to-target strategies.
Efficacy and Safety Aspects: What is the Best Approach?
Professor Julián Panés
Prof Panés described the efficacy and safety aspects of biologic therapies. He began by discussing the clinical case of a 55-year-old male patient with colonic CD; the patient had showed some improvement with prednisone treatment, but remission had not been achieved at 4 weeks of treatment. The patient was subsequently started on adalimumab as monotherapy and tapered off corticosteroid treatment. The patient showed sustained improvement following initiation of adalimumab, but still had persistent mild-to-moderate symptoms. In the 6-month period following adalimumab initiation, the patient experienced several infections, after which he stopped adalimumab therapy and switched to ustekinumab. He achieved clinical remission at Week 8 of treatment, and sustained remission through the next 5 years of treatment with no serious infection-related adverse events (AE).
Data from the UNITI-1 and UNITI-2 trials in patients with CD showed that ustekinumab treatment is associated with similar rates of AE, severe AE, and infections through Week 8 of treatment versus placebo.14 The safety profile for ustekinumab remained consistent through Week 156 of treatment in the IM-UNITI trial.15,16 Furthermore, data from the UNIFI trial in patients with UC show that through 1 year of treatment, rates of key safety events remained similar between patients who received placebo and those who received ustekinumab.8 The safety profile of ustekinumab remained consistent through 1 year of treatment across other indications (including psoriasis and psoriatic arthritis), and was comparable to placebo in registrational trials including approximately 6,000 patients.17
The Use of Treatment Monotherapy Versus Combination Therapy
Professor Julián Panés
Compared with monotherapy, the use of combination therapy in IBD has been associated with an increased risk of AE;18,19 however, data from the IM-UNITI trial showed that rates of antidrug antibody formation remained low through Week 156 in patients with CD treated with ustekinumab.15 Furthermore, data from the UNIFI induction and maintenance trials revealed that antidrug antibody formation rates were low through Week 8 in patients with UC who were treated with ustekinumab.20,21
In patients with IBD, data from the IM-UNITI and UNIFI trials also showed that concomitant use of immunomodulators does not appear to affect the efficacy of ustekinumab treatment in patients with IBD, suggesting that, with ustekinumab, there is no need for combination therapy. Remission efficacy in patients with CD was maintained through Week 92 of treatment, regardless of whether patients were receiving concomitant immunomodulators.13,22 Furthermore, immunomodulator use did not affect serum ustekinumab concentrations in patients with CD though Week 92 of treatment.23
Prof Vermeire noted that monitoring of drug levels may not be necessary if patients are still showing a clinical response, though patients who are losing response may require monitoring for possible dose optimisation. In conclusion, Prof Vermeire reiterated the importance and application of treat-to-target strategies in UC and CD management, stressing the importance of the current data regarding safety and efficacy with biologic treatments, and the lack of added outcome benefits with combination therapies.








