Meeting Summary
Biosimilars have contributed substantially to the evolving therapeutic landscape for inflammatory bowel disease (IBD), enabling healthcare systems to offer access to a broader range of therapies at an affordable price. Despite the increasing confidence healthcare practitioners (HCP) place in the safety and efficacy of biosimilars, uncertainty remains around best practice when switching a patient from a reference product to a biosimilar. This symposium aimed to uncover the importance of a managed switch programme by exploring real-world data from the University Hospital Southampton NHS Foundation Trust, Southampton, UK, and University Hospital Erlangen, Erlangen, Germany. The roles of therapeutic drug monitoring and predictive outcome scoring were discussed based on evidence from the SECURE and the CREOLE studies, respectively. Finally, in light of the recent European Union (EU) approval of adalimumab biosimilars, the future therapeutic landscape and how physicians and patients can make well-informed decisions when multiple versions of the same biologic are available were discussed.
Introduction
Prof Van Assche moderated a highly interactive podium discussion between the faculty members and the audience, addressing key clinical points around switching patient treatment to a biosimilar. Interactive technology was used to maintain continuous dialogue with the audience.
Since the introduction of infliximab almost two decades ago, anti-tumour necrosis factor (TNF) biologics have revolutionised treatment for IBD. Several other anti-TNF biologics have followed, in addition to two alternative agents with novel mechanisms of action: vedolizumab, an integrin antagonist that targets the gut-specific α4β7 integrin for inhibition,1 and ustekinumab, which targets the upstream regulatory cytokines interleukin-12 and interleukin-23 to disrupt the inflammatory cascade.2 Recent approvals of biosimilars have also widened treatment options by providing cost-effective alternatives to reference products. To date, there are >20 biosimilar products licensed in Europe, including biosimilars for infliximab (e.g., CT-P13 and SB2) and etanercept (e.g., SB4 and GP2015). According to Prof Van Assche: “Anti-TNF will be a mainstay treatment for the next 10 years for IBD. Additionally, with adalimumab biosimilars and oral therapeutics on the horizon, gastroenterologists will be empowered with exceptional choices when selecting the best treatment for their patients with IBD.” With several years of real-world experience now supporting confident use of biosimilars, the practicalities of introducing biosimilars effectively into clinical practice have come to the fore; indeed, when the audience was asked whether they already used biosimilars in their daily clinical practice, half of the attendees reported that they were, with approximately 60% of the remaining half confirming they were happy to switch their patients to a biosimilar.
Best Practices and Key Considerations for Switching: Lessons from Case Studies
A managed switch programme using a gainshare model can deliver significant cost savings and investment in clinical services while maintaining comparable clinical responses, patient-reported outcomes (PRO), and drug persistence. As Dr Cummings explained: “In a gainshare model, you make an investment or a change in your practice and the benefits are shared between the different stakeholders involved in the process, therefore including the payers, the hospital, the clinical team, and the patients.” Dr Fraser Cummings, Prof Raja Atreya, and Prof Geert D’Haens shared their personal experience, discussing the key elements that made their programmes successful (Figure 1).
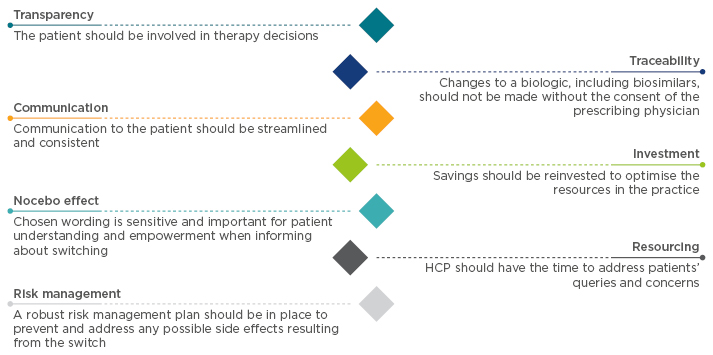
Figure 1: Best practices to create a reference biologic-to-biosimilar switching model.
HCP: healthcare practitioner.
At Southampton General Hospital, Southampton, UK, Dr Cummings and his team switched 143 IBD patients from originator infliximab to CT-P13.3 Dr Cummings summarised: “We saw no change in immunogenicity or in objective inflammatory markers; in general, about 20% of patients on infliximab stop treatment within a calendar year, so it was important that we demonstrated no difference in the drug persistence rate after we switched patients. We showed a very clear decrease in drug acquisition costs and astatistically significant improvement in patients’ reported quality of life. The latter could beattributed to the additional resources that we acquired as a direct result of the cost savings.”
Similarly, Prof Atreya participated in the implementation of a gainshare model in his department at University Hospital Erlangen, Erlangen, Germany, where 119 patients with ulcerative colitis (UC) or Crohn’s disease (CD) were successfully switched from reference infliximab to SB2 between February and April 2017.4 Prof Atreya reported: “6 months after switching, most patients remained in clinical remission, having a Mayo score of 0 or 1 for patients with UC, and Harvey–Bradshaw index <5 for patients with CD, with no statistical significance in the changes in clinical outcomes compared to Week 0. Median trough levels measured through therapeutic drug modelling (TDM) revealed no statistically significant change from baseline to Week 24.”
Likewise, Prof D’Haens and his team successfully switched 100 patients with CD from reference infliximab to biosimilar CT-P13 in the SECURE study,5 which assessed serum drug concentration and clinical activity. “There was no difference in the serum concentration of CT-P13 at Weeks 8 and 16 following the switch compared with reference infliximab. In terms of immunogenicity, the signals were very reassuring: C-reactive protein values remained close to zero, despite a slight variation, which is to be expected. These favourable outcomes prompted switching of 95% of patients at the institute to a biosimilar.”
Key Aspects of Patient Communication
When discussing effective ways to involve patients in shared decision-making regarding switching, Dr Cummings reported: “At a very early stage we brought in a patient panel, which is a group of people that meet with us every month or couple of months to discuss the service and the care that we are delivering. They are also very useful for discussing ideas and research proposals. We spent a couple of hours discussing a potential biosimilar switch with our patient panel and this was key to the successful outcomes of our switching process. Patients were thus able to make informed decisions and become an integral part of the switching model. Physicians used this time to develop new ideas to improve their research, delivery of care and the process they were putting in place, to effectively develop the switching programme.”
During the programme, Dr Cummings identified that: “Infusion nurses as well as specialist nurses play a critical role as information providers. Patients have the most concerns at the time preceding and during the switch to a biosimilar, and often the infusion nurses are the HCP that spend some time addressing patients’ feelings and concerns.” Infusion nurses were, therefore, educated about biosimilars and allowed to have a space where their potential concerns about switching could be addressed, in order for them to both understand and be confident in the use of biosimilars, and also transfer this confidence to the patients. Dr Cummings explained: “It is important that the nurses are not anxious about switching, as HCP communicate with the patients both verbally and with body language.”
“Another key source of information are physicians. At the departmental meeting, physicians were able to address any queries surrounding reference originator-to-biosimilar switching. Communication with patients was thus streamlined to ensure patients received the same message from all parties. Additionally, HCP were reassured by a robust risk management plan so that if there was a problem in switching patients, they would find it quickly and be able to react appropriately. This gave HCP the confidence to engage in the plan and feel reassured about their patients’ care,” Dr Cummings stated.
During the same programme, as additional support, patients were given a letter to explain what a biosimilar is. Dr Cummings explained that: “Patients were able to discuss the contents of the letter with the specialist nurses and had time to consider its contents at home. Patients then confirmed whether they were happy to go ahead with the switch at their next infusion appointment.”
Addressing the Nocebo Effect
Physician-to-patient communication is also key to reducing the likelihood of a nocebo effect. The nocebo effect is the negative equivalent of the placebo effect, and has historically been shown to have a detrimental impact on treatment adherence and outcome in several diseases.6 Negative perceptions due to lack of understanding and knowledge about biosimilars can result in a nocebo effect;7 therefore, minimising the nocebo effect by increasing knowledge and understanding about biosimilars is crucial when switching patients from a reference biologic to a biosimilar. “Patients who are in remission following a very severe disease and receiving a reference biologic are particularly reluctant to change to a biosimilar. Physicians are aware that with all TNF inhibitors there will be a loss of treatment response over time, but for patients, once they switch, they assume that the loss of response is due to the biosimilar,” Prof Yoram Bouhnik stated.
Dr Cummings suggested a way to overcome this: “Prior to the switch, discuss the side-effects patients might already be experiencing and potential flares on the reference drug with patients; this provides an opportunity to reduce possible nocebo effects following the switch to biosimilar.” According to Dr Cummings, the implementation of this element in his switch programme was associated with the lack of changes in side effects reported when patients were switched to the biosimilar.
Reinvesting Savings into the Clinic
If the nocebo effect is minimised, uptake and adherence to biosimilars can be improved and ultimately result in savings for departments and healthcare systems. These savings can then be realised within the department if a gainshare model is used; i.e., an agreement that some or all of the savings made are retained within the department initiating the change, as Dr Cummings clarified. He added: “This scheme worked because all the key participants benefited from the model; in our case, this included first and foremost the patients, but also the institutions, the pharmacy, the payers, and the faculty.”
“A key element for the success of a gainshare model is the clear incentivisation to the clinic providing the services so that these are implemented and acknowledged,” stated Dr Cummings. In his case, the switch to biosimilars led to a reduction in drug acquisition costs of £40,000–60,000 per month. “My team was highly incentivised, as the savings were used to acquire an additional IBD nurse, as well as a secretary to support both the physicians and the nurses. This definitely contributed to making the model a success.” Dr Cummings believes: “It is always important to acknowledge the amount of work involved when switching patients… …but the incentivisation depends on the healthcare system. Switching to biosimilars takes time, energy, and resources, and that is part of the reason why we made sure to get our share reinvested in our service. The significant increase in patient outcome measures noted in the programme could be attributed directly to the cost savings with investment in more resources for patients. To this point, it is crucial to notice how the uptake of biosimilars has been much lower in models where the share was not reinvested in the institution.”
Similarly, Prof Atreya believes the success of the switch at University Hospital Erlangen can be attributed to the use of a gainshare model, the financial benefit of which enabled his clinic to hire a second physician in the IBD unit: “As switching is an emotional process for patients, the availability of a second physician enabled us to spend more time with patients and introduce them comprehensively to the topic of switching in a much more relaxed manner. This was particularly important for the patients who have been in remission on the reference biologic for many years. Therefore, the gainshare model benefited both patients and HCP within the department.” Prof Atreya has had experienced patients querying whether the switch in treatment is solely to benefit the hospital financially: “Patients can wrongly perceive the switch to a less expensive product as a switch to a less effective and potentially more harmful medication. It is important to highlight to the patient how this switch in treatment leads to the financial benefits to the hospital that are then utilised within the department, such as the addition of new staff, more resources to support patients through the switch process, and shorten waiting times for clinical visits. This allows the patient to experience the benefits directly.” As a result of the success of his model, Prof Atreya confirmed that no patient had left the institute to seek care at another institute, and concluded: “Taking the time to explain to patients and paying attention to their worries is of outmost importance.”
Results from a symposium polling question revealed that the biggest obstacle in implementing a gainshare model within an institute was that the cost savings could not be retained by the department. To ensure that the savings would be invested into the department and not in the hospital cost improvement programme that is in place in the UK, Dr Cummings established very strong relationships with the payers and the hospital management at a very early stage. He argued: “The financial success can only come true if the investors understand that using this gainshare model to develop a biologic service will be both cost effective and cost efficient, and will provide the patients with higher-quality care. The gainshare model should, therefore, be pitched as a model that can save you money and provide better care.”
Switching Between Biosimilars
The implementation of a successful reference biologic-to-biosimilar switch model can result in significant cost savings for the department. However, with an ever-widening IBD treatment armamentarium, clinics need to also start considering best practices surrounding a biosimilar-to-biosimilar switch. When asked, the audience revealed mixed results relating to their confidence in terms of a biosimilar-to-biosimilar switch. Prof D’Haens shared that he had previously performed a second switch; however, he explained: “Both biosimilars were manufactured in the same factory, and hence were inherently the same; had these been produced in a different environment, I would have been unsure.”
According to Prof Atreya, “if we see no ‘red flags’ on the first switch, why should we expect any surprises on the second?” In agreement, Dr Cummings clarified: “Intellectually, switching to another biosimilar should be no different than switching from a reference biologic to another biosimilar. It is important to acknowledge, however, that regulatory bodies, such as the European Medicines Agency (EMA), usually compare the reference biologic to the biosimilar and not the biosimilar to another biosimilar. Recent data suggest that there are no differences between biosimilars at the characterisation level. Additional data have demonstrated cross-reactivity between SB2, CT-P13, and between both of them and the reference biologic;8 we can therefore assume that the results could be extrapolated into clinical practice and that there would not be a problem. Data coming out in the coming year will hopefully confirm this theory and unveil the feasibility of a biosimilar-to-biosimilar switch, or potentially a biosimilar-to-reference biologic switch, should the cost permit it.”
Using Therapeutic Drug Monitoring
Regardless of the type of switching, therapeutic drug monitoring (TDM) can be a helpful tool to monitor patients. In a symposium poll, 53.2% of the audience responded that they use TDM to optimise biologic effectiveness in specific patients, while 19.1% replied that they use it regularly.
During the SECURE study, Prof D’Haens used TDM to monitor the switch from infliximab to CT-P13: “TDM was introduced in our SECURE study to compare serum concentrations, presence of antidrug antibodies and C-reactive protein levels between reference biologic and biosimilar, and to provide patients with additional support when considering switching. The data gave the patients further confidence that switching to a biosimilar is a safe and viable approach.” Prof D’Haens commented: “We used TDM extensively and intensively during the first year of the study but have used it less as our confidence in switching increased.”
Predictive Factors: Future of Treatment Success?
Predictive factors that could forecast the success of biologic therapy (regardless of whether a reference product or biosimilar) in biologic-naïve patients could significantly impact the patient’s quality of life, allowing the patient to receive the most appropriate treatment sooner. Patients with CD and stricture, the most common complication in CD patients, were initially contraindicated for anti-TNF treatment due to the increased risk of intestinal obstruction. Treatment of this condition is difficult, and these patients are often recommended for surgery. However, evidence from the TREAT and the ACCENT registries9 suggests that infliximab treatment is not associated with an increased risk of obstruction in patients with or without intestinal strictures at baseline. Prof Bouhnik therefore initiated the CREOLE study10 to establish the effectiveness and safety of adalimumab in patients with CD and symptomatic small bowel stricture.
In this study, 97 patients were included and received treatment with adalimumab. The primary endpoint, defined as adalimumab success at Week 24, was observed in 62 of 97 patients. Moreover, multivariate analysis found seven clinical and imaging parameters that were independently associated with the success of adalimumab treatment, and could therefore be used as predictive markers (Figure 2). To calculate the CREOLE score, one point should be given to each factor: patients with a score >3 will most likely succeed under adalimumab treatment (>90%), while patients with a score <2 (<10%) should instead be treated with surgery.
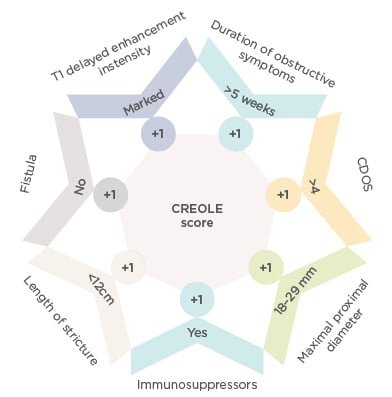
Figure 2: Predictive markers to calculate the CREOLE score.
CDOS: Crohn’s Disease Obstructive Score.
Prof Bouhnik reported: “The use of the CREOLE score as a prognostic factor was associated with >50% of patients initially treated with adalimumab being surgery-free after a median follow-up of 4 years; however, an independent study is required to validate these innovative results.”
Biosimilars in the Future
“The upcoming adalimumab biosimilars, which will be available as a subcutaneous injection, as opposed to an intravenous infusion, will have a significant impact in the field of biosimilars,” predicted Prof Atreya. “However, in terms of safety and efficacy, the new biosimilars will probably perform as well as their reference biologic, in the same way that infliximab biosimilars had performed as well as infliximab; all biosimilars have to fulfil a very diligent comparability exercise and clinical tests before entering the market. The validity of these tests has been shown by infliximab biosimilars; therefore, if the new biosimilars are able to pass their preclinical tests, then we can expect them to be functional clinically.” A good example is the biosimilar SB5. “The data11,12 are very reassuring, as they reveal no clinically meaningful differences in terms of pharmacokinetics, safety, and efficacy,” Prof D’Haens stated.
Conclusion
Biosimilars have been shown to have similar critical quality attributes, such as physicochemical qualities and Fab/Fc-related biological activity, equivalent pharmacodynamics and efficacy, and similar safety profiles to their reference biologic. Accumulated evidence from real-world experience with biosimilars has confirmed biosimilarity, leading to an increase in physician confidence in their understanding and use. However, further information on how to implement biosimilars into their clinic efficiently and effectively is required.
The case studies discussed during this meeting highlight that the implementation of a switching programme can offer substantial benefits to both the institute and the patients. Prof D’Haens concluded: “The three key factors that make a switching programme both a financial and a clinical success are transparency: always tell the patients what you are doing, and what you know; traceability: always know what the patient received and when; communication: ensure that everyone is telling the same story.” With careful planning, financial benefit can be reinvested in the practice to result in additional resources and support, which will directly benefit all stakeholders.








