Summary
TNF-α is produced in high concentrations in chronic inflammatory disease, resulting in excessive inflammation which eventually leads to organ damage. The advent of anti-TNF therapy in clinical practice 20 years ago represented a significant change in the management of immune-mediated inflammatory diseases (IMIDs), including rheumatoid arthritis (RA), axial spondylarthritis (SpA), psoriasis, and inflammatory bowel disease (IBD).
There are five anti-TNFs approved for use in IMIDs: infliximab, adalimumab, golimumab, etanercept, and certolizumab pegol. The structural and pharmacological differences between these agents mean that they can have differential efficacy across IMIDs, and therefore the indications for which they are approved vary. This mini-review aims to summarise the current understanding of anti-TNF efficacy in those IMIDs for which they are approved, focussing on data from meta-analyses of randomised clinical trials (RCTs), and real-world studies.
INTRODUCTION
Between 5% and 7% of the western population are affected by IMIDs, including RA, SpA, juvenile idiopathic arthritis (JIA), non-infectious uveitis, inflammatory skin conditions such as psoriasis and hidradenitis suppurativa (HS), and IBDs such as Crohn’s disease (CD) and ulcerative colitis (UC).1,2
IMIDs span the fields of gastroenterology, rheumatology, and dermatology, with an increasing understanding of the overlapping pathogenesis between them. As such, patients with one IMID have an increased risk for developing others,3 and IMIDs are now primarily treated according to their pathological mechanism rather than with organ-based symptomatic relief.4
ANTI-TNFS AS A THERAPY FOR IMMUNE-MEDIATED INFLAMMATORY DISEASES
In chronic inflammatory diseases, the cytokine TNF-α is produced in high concentrations, leading to excessive inflammation and eventually organ damage.5 Anti-TNFs bind to TNF-α to inhibit its interaction with the TNF receptor, resulting in downregulation of pro-inflammatory cytokines and adhesion molecules, increased circulation of regulatory T cells, and reduced migration of inflammatory cells into inflamed tissue.6 Extra-immune activity of anti-TNF includes the attenuation of angiogenesis and vascular permeability, and deactivation of endothelial, epithelial, and mesenchymal cells,6 suggesting a role in reducing tissue inflammation.
The advent of anti-TNF therapy has led to significant progress for patients with chronic inflammatory disease,7 and to date, there are five anti-TNFs approved for use in IMIDs: adalimumab, infliximab, golimumab, etanercept, and certolizumab pegol.8-13 This mini-review aims to summarise the current understanding of the efficacy of this drug class in IMID, focusing on data from meta-analyses and real-world studies due to the vast amount of data produced by individual clinical trials (Figure 1).
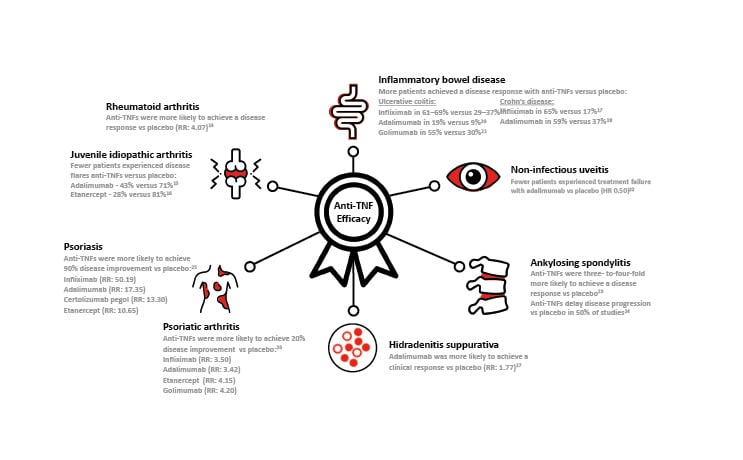
Figure 1 : Anti-TNF Efficacy in Immune-mediated disease.*
*Efficacy values are taken from selected articles reviewed in the main text. HR: hazard ratio; RR, risk ratio; TNF:tumour necrosis factor.14-27
EFFICACY OF ANTI-TNF MEDICATION
Given the overlapping pathogenesis between IMIDs, one might expect anti-TNF agents to be similarly effective. However, structural and pharmacological differences between these agents mean that they can have differential efficacy across these diseases.28 For example, while infliximab, adalimumab, and golimumab are whole antibodies that bind bivalently to TNF and form multimeric complexes, etanercept and certolizumab pegol bind monovalently to TNF.28
Rheumatoid Arthritis
All five approved anti-TNFs have been shown to be efficacious in the treatment of RA. Several systematic reviews and meta-analyses have been conducted to bring together the vast amount of data that supports the efficacy of anti-TNFs in this disease.
Aaltonen et al.14 analysed 26 RCTs showing that anti-TNFs were more efficacious than placebo at all time points, achieving a relative risk (RR) of achieving American College of Rheumatology (ACR) 50 (≥50% improvement in the ACR disease activity score) of 4.07. The highest risk was associated with etanercept (RR: 8.61), and the lowest with infliximab (RR: 3.08), and golimumab (RR: 1.56). This analysis also found that the combination of an anti-TNF with methotrexate was superior to either anti-TNF or methotrexate alone.
To compare the efficacy of anti-TNF plus methotrexate combination therapy with triple therapy (methotrexate plus hydroxychloroquine plus sulfasalazine) in the treatment of RA, Fleischmann et al.29 conducted a meta-analysis of 33 RCTs. They reported that in patients with an inadequate response to methotrexate monotherapy, triple therapy was associated with lower odds of achieving ACR70 at six months (odds ratio [OR]: 0.35) compared with an anti-TNF plus methotrexate.
Focusing on the inhibition of radiographic progression in RA, Murray et al.30 compared the efficacy of different biologics. Nine studies published were included in the meta-analysis. Four of the five anti-TNFs, when combined with methotrexate, were associated with a statistically significant reduction in radiographic progression compared with methotrexate alone. Adalimumab plus methotrexate achieved the largest mean difference in van der Heijde modified Sharp (vdHS) score versus methotrexate alone (3.8 fewer units), followed by infliximab plus methotrexate (3.7 fewer units). However, in this analysis, golimumab plus methotrexate was not associated with a significant effect on radiographic progression compared with methotrexate alone.
Juvenile Idiopathic Arthritis
JIA is a heterogenous group of chronic inflammatory arthritic diseases with an onset before the age of 16.31 Three anti-TNFs are approved for use in the polyarticular sub-type of JIA (pJIA): adalimumab, golimumab, and etanercept,9-11 although infliximab is also widely used off-label.31
Adalimumab, alone or in combination with methotrexate, was shown to be efficacious in patients aged 4–17 years with active pJIA.15 This RCT used a withdrawal design to minimise the numbers of children exposed to placebo. All participants (N=171) received adalimumab for 4 months, and responders (n=133) were then randomised to receive adalimumab or placebo for up to 8 months. Of those participants not concurrently taking methotrexate, 43% of those receiving adalimumab experienced disease flares versus 71% of those receiving placebo (p=0.03). Of participants who were taking methotrexate, 37% of those receiving adalimumab experienced disease flares versus 65% of those receiving placebo (p=0.02).
In a similarly designed RCT, 28% (7/25) of participants receiving etanercept experienced a disease flare during the study, compared with 81% (21/26) of patients receiving placebo (p=0.003).16 The median time to a disease flare was significantly longer with etanercept versus placebo (116 days versus 28 days; p<0.001).16
Golimumab has also been shown to result in rapid improvement. In the open-label, lead-in phase of a study in 173 patients aged 2–17 years with active pJIA, golimumab treatment resulted in disease improvement as early as Week 4, with 34.1% of patients achieving clinically inactive disease. Eighty-nine percent of patients (n=154) achieved an ACR30 response in this lead-in phase, and were randomised to receive golimumab or placebo for 48 weeks or until they experienced a disease flare. However, at Week 48, disease flares were comparable between treatment groups (golimumab: 41% versus placebo: 47%), resulting in failure to meet the primary endpoint of the study, which was the proportion of patients without a disease flare at Week 48. It has been suggested that this may have been due to a carry-over effect from golimumab treatment in the lead-in phase.32
Crohn’s Disease
Infliximab, adalimumab, and certolizumab pegol are all approved for use in CD (certolizumab pegol only in the USA)8,9,13 and represent a central treatment modality for IBD.33
Targan et al.17 showed that in patients with moderate-to-severe CD, 4 weeks of infliximab treatment produced a clinical response rate of 65% (54/83) versus 17% (4/24) with placebo. Several years later, four weeks of adalimumab treatment was shown to induce an improvement of symptoms in 59% of patients versus 37% with placebo (n=299) in the CLASSIC-I study.18
Beyond induction of a clinical response, the efficacy of anti-TNFs in maintaining a response in CD has been demonstrated. The ACCENT I study showed that 30 weeks of infliximab therapy resulted in clinical remission in 39% of infliximab responders with luminal CD, versus 21% of those receiving placebo at the same time points. After Week 54, clinical remission had been achieved in approximately 30% of responders receiving infliximab versus approximately 15% of those receiving placebo.34 A real-world, long-term (approximately 4.5 years) study supported these findings, reporting that infliximab resulted in 63.4% (n=347) of responders achieving sustained clinical benefit.35 Adalimumab (40 mg weekly) was shown to maintain remission in patients with moderate to severe CD in the CHARM study, with 41% of 4-week responders achieving remission at week 56 compared with 12% of those receiving placebo.36
Both infliximab and adalimumab are also approved for use in paediatric patients aged ≥6 years or above with CD.8,9
Ulcerative Colitis
Anti-TNFs have become the cornerstone of treatment for moderate-to-severe UC, both improving quality of life and decreasing the risk of hospitalisation.37 Infliximab, adalimumab, and golimumab are all approved for use in UC.8-10
The large ACT 1 and ACT 2 studies (each n=364) in patients with moderate-to-severe active UC showed that infliximab treatment produced a clinical response in 61–69 % of patients at Week 8, compared with 29–37% of those receiving placebo (p<0.001 in each study).19 Pooled data from the long-term extensions to these two studies (n=229) showed that the proportion of patients with no or minimal disease was maintained for up to 3 years following the initial studies, from 76.5% at extension Week 0 to between 90.0% and 94.3% at extension Week 152.38
The ULTRA 2 study demonstrated the benefits of adalimumab for moderate-to-severe UC (n=494), showing that clinical remission was achieved in 16.5% of patients treated with adalimumab versus 9.3% of those treated with placebo at Week 8, and in 17.3% versus 8.5% of patients, respectively, at Week 52.20 A real-world study supported these findings, showing that clinical remission with adalimumab was achieved by 54.9% (56/102) of patients at 3 months, and 56.6% of patients at a median (95% confidence interval [CI]) of 18 (12–24) months.39
The PURSUIT studies supported the use of golimumab in UC.21,40,41 PURSUIT-SC reported rates of clinical response at Week 6 of 54.9% in patients treated with subcutaneous golimumab (400/200 mg) versus 30.3% of those receiving placebo (n=774).21 PURSUIT-IV reported similar rates of clinical response for intravenous golimumab.40 Longer-term maintenance with golimumab was demonstrated by the PURSUIT-M LTE study, which showed that among responders who remained on treatment to the end of the extension study (n=134), 99.3% (133/134) had no or mild disease activity at Week 216.41
Both infliximab and adalimumab are also approved for use in paediatric patients with UC aged ≥6 years and ≥5 years, respectively.8,9
Non-infectious Uveitis
Intraocular inflammatory disorders like uveitis are associated with visual morbidity and sight loss;42,43 resulting in blindness in up to 22% of patients.43 Anti-TNFs have revolutionised therapy for uveitis and are increasingly used in clinical practice.42 To date, the only currently approved anti-TNF for this indication is adalimumab,9 though infliximab and etanercept have been used off-label.44
The adalimumab studies leading to approval for use in uveitis were VISUAL I and VISUAL II.22,45 VISUAL I demonstrated that patients with active, prednisone-refractory uveitis (n=217) who received adalimumab were less likely to experience treatment failure compared with patients who received placebo (hazard ratio [HR] 0.50; p<0.001).22 VISUAL II showed that adalimumab was also effective in patients with inactive, prednisone-controlled uveitis (n=226), of whom 39% experienced treatment failure compared with 55% of patients receiving placebo.45 In these studies, 37% of uveitis cases were idiopathic, and 68% were associated with other IMIDs
Adalimumab is also approved for use in paediatric patients with uveitis aged ≥2 years.9
Axial Spondyloarthritis
Axial SpA is an inflammatory arthritis that predominantly affects the sacroiliac joints and spine, resulting in stiffness, pain, and eventually spinal fusions.46 Axial SpA can be divided into two subtypes: ankylosing spondylitis (AS), in which structural changes to the sacroiliac joints can be detected on radiographs, and non-radiographic axial SpA.46 All five anti-TNF agents are approved for AS, 8-13,46 and some are also approved for use in non-radiographic axial SpA by certain regulatory authorities.9,11-13
A meta-analysis in 2015 assessed the benefits of anti-TNFs in AS across 21 short-term (≤24 weeks) RCTs and reported high-quality evidence that patients on anti-TNFs were three-to-four-fold more likely to achieve an Assessment in Spondyloarthritis International Society composite score of 40% (ASAS40) response at 6 months compared with placebo: infliximab (RR: 4.07); adalimumab (RR: 3.53); etanercept (RR: 3.31); and golimumab (RR: 2.90).23 Certolizumab pegol was not assessed as it was not approved for use in AS at the time of the analysis.12,13
A more recent meta-analysis assessed the effect of anti-TNFs on radiographic progression in AS across 21 studies.24 Fifty percent of the studies demonstrated a delay in radiographic progression compared to placebo, and this was particularly evident in studies with >4 years of follow-up (80%) compared with studies with ≤4 years of follow-up (27% of studies). 24
To investigate characteristics of patients with AS that might predict response to anti-TNFs, a recent retrospective cohort study pooled data from 10 RCTs.47 The probability of a major response (a decrease in AS Disease Activity Score [ASDAS] of ≥2) was found to increase with higher C-reactive protein levels and disease activity scores, and decrease with age, findings which may facilitate personalised treatment decisions in the future.
Psoriasis
Psoriasis affects 1.5% of the Western European population, and commonly presents in middle age (incidence increases up to 39 years of age and then decreases).48 Infliximab, adalimumab, etanercept, and certolizumab pegol are all approved for use in psoriasis to improve symptomatic control.8,9,11-13 In addition to the characteristic erythematous plaques, psoriasis has been associated with comorbid conditions such as metabolic syndrome, IBD, cardiovascular disease, and psoriatic arthritis (PsA).49
A recent network meta-analysis of 167 studies reported the proportion of patients with psoriasis that reached 90% improvement on psoriasis area and severity index (PASI90) during the induction phase (8–24 weeks after randomisation) of various systemic treatments compared with controls.25 Of all the anti-TNFs assessed, infliximab was found to have the highest probability of leading to PASI90 relative to placebo (RR: 50.19), followed by adalimumab (RR: 17.35), certolizumab pegol (RR: 13.30), and etanercept (RR: 10.65).25 Another meta-analysis reported the efficacy of anti-TNFs with longer-term treatment, the number needed to treat to achieve PASI90 at 48 or 52 weeks was reported as adalimumab (2.17) certolizumab pegol (1.72), and etanercept (3.20).50
Adalimumab and etanercept are also approved for use in paediatric patients with psoriasis.9,11
Psoriatic Arthritis
PsA affects up to 30% of patients with psoriasis and can present in a variety of ways, including joint pain and swelling, enthesitis, and dactylitis.49 All four of the anti-TNFs approved for use in psoriasis are also approved for use in PsA, with the addition of golimumab.8,9,11-13
In a systematic review and network meta-analysis of 25 studies, Ruyssen-Witrand et al.51 found that anti-TNFs, particularly infliximab, golimumab, and etanercept, were the most effective biological agents in PsA in terms of joint symptoms and that infliximab was the most effective in terms of skin symptoms. An earlier meta-analysis reported the efficacy (in terms of the probability of achieving ACR20 versus controls) of anti-TNFs as: adalimumab (RR: 3.42); etanercept (RR: 4.15); golimumab (RR: 4.20); and infliximab (RR: 3.50).26 Certolizumab pegol (200 mg twice weekly) has been shown to achieve ACR20 in 58.0% of patients with PsA over 12 weeks of treatment compared with 24.3% of those receiving placebo (p<0.001; n=368).52
Hidradenitis Suppurativa
Adalimumab is currently the only anti-TNF approved for HS, a painful inflammatory skin disease.9,53 The efficacy of adalimumab was shown to be effective in HS in two Phase III clinical studies, PIONEER I and II, which reported that clinical response rates at Week 12 were significantly higher in patients receiving adalimumab versus those receiving placebo (PIONEER I [n=307]: 41.8% versus 26.0%; p=0.003; PIONEER II [n=326]: 58.9% versus 27.6%; p<0.001).53
More recently, a meta-analysis of five RCTs found that adalimumab treatment versus placebo was associated with better clinical response achievement (RR: 1.77) and dermatology life quality index (DLQI) score.27
A small, real-world study supported these findings, showing that of 19 patients with HS treated with adalimumab, 10.5% achieved a clinical response at Week 4, 42.1% at Week 12, and 63.2% at Week 24.54
LOSS OF RESPONSE
Anti-TNFs have revolutionised the treatment of many IMIDs, making remission an achievable goal.55 However, roughly half of patients that initially respond to anti-TNF therapy will experience a gradual loss of response over time.6,36,55,56 This is thought to result primarily from the development of anti-drug antibodies which reduce circulating anti-TNFs to sub-therapeutic levels.55,56
To address this issue, a treat-to-target strategy is increasingly applied to IMIDs, including therapeutic drug monitoring, anti-TNF dose intensification, tapering, switching to other biologic agents, and/or combination with immunomodulators.6,55 A detailed discussion of these approaches lies beyond the scope of this article and has been presented in a number of recent review articles. 6,36,55,56
CONCLUSION AND SUMMARY
Anti-TNFs have transformed the management of IMIDs since their introduction more than 20 years ago.8 The literature shows that they continue to form the mainstay of treatment for many of these conditions, and research is progressing in other indications for which they may prove beneficial.
The loss of response to anti-TNFs experienced by many patients is managed using therapeutic drug monitoring and treat-to-target strategies, which will be supported by ongoing research into biomarkers of IMIDs.6
For both clinicians and their patients, pharmacovigilance and personalised medicine are the best methods for optimising anti-TNF therapy.57








