Abstract
Chronic inflammation is the single major contributor to the pathogenesis of sigmoid colon inflammatory diseases such as segmental colitis associated disease and inflammatory bowel disease (IBD). Existing conventional anti-inflammatory treatments have not proven to be a sufficient long-term solution for management of symptoms due to the immunosuppressive nature of these agents. Stem cell (SC) transplantation is a novel approach to treatment that could improve the prognosis of IBD patients in the long term by preventing inflammation, restoring defective immune balance, and promoting mucosal healing. Multiple studies have shown that bone marrow SC, mesenchymal SC (MSC), and most recently intestinal SC (ISC) have had marked success in improving immune functionality in cases of IBD. Effects of bone marrow SC did not show the kind of longevity that researchers initially anticipated, leading them to instead pursue thorough study of MSC. The tolerogenic effects of MSC have proven them to be a key player in the development of SC therapy; however, their exact mechanism of action has yet to be fully characterised. Due to existing discrepancies in the data detailing the association between MSC and colorectal cancer risk, ISC have since become of interest with the intention of finding a more reliable alternative source of SC. Preliminary studies have shown that ISC may be capable of achieving the same immunomodulatory effects as MSC but with reduced colorectal cancer risk, suggesting them to be the most promising new method of treating inflammatory-based sigmoid colon diseases under study thus far.
INTRODUCTION
The authors of this review aim to provide the latest information on the correlation between immunopathology of sigmoid colon inflammatory diseases and the application of innovative therapies using stem cells (SC). The anatomical approach, regarding the sigmoid colon, is based on the overlap of many diseases of this part of large colon either in their histopathological diagnosis and treatment or their epidemiological trends and risk factors.
Characteristic diseases of the sigmoid, such as diverticulitis, segmental colitis associated disease, and inflammatory bowel disease (IBD) have a common hallmark: chronic inflammation. The problematic inflammatory process taking place in the gastrointestinal (GI) tract is the deleterious effect of an otherwise well-balanced host defence system.
This review article presents the immune system mechanisms of the GI tract, molecules associated with chronic inflammation, and new options of treatment based on the immunomodulatory properties of the haematopoietic (HSC) and mesenchymal stem cells (MSC) transplantation or transfusion, as well as intestinal stem cells (ISC) grown in vitro used as donor cells for transplantation, which are termed organoids.
SC treatment for IBD has recently attracted much interest and generated numerous publications. Among the different inflammatory diseases of the sigmoid colon these innovative treatment solutions with SC, which are in different phases of clinical research, are analysed mainly in patients with ulcerative colitis (UC). In other words, the authors will draw upon the present articles on UC to give the latest information on the current knowledge regarding this new treatment. Experimental work has yielded encouraging results; however, several aspects remain unresolved. The complexity of the issues involved raises many questions requiring further study and clarification.
This article is a comprehensive presentation of the most relevant data available in the existing literature emphasising the pathways of these multifaceted and complex interactions.
FUNCTIONAL FEATURES OF SC IN THE INTESTINAL EPITHELIUM
The sigmoid mucosa is covered by a single columnar epithelium that form one layer of cells linked together with tight junctions. These cells, enterocytes, goblet cells, and neuroendocrine cells, have a common progenitor: the so called ISC LGR5+, also known as crypt base columnar cell (CBC).1 SC reside at the base of the crypts, forming a small niche. Under normal conditions the intestinal epithelium has a renewal rate every 3–8 days.2
SC are capable of differentiating into many mature types of intestinal epithelial cells, regenerating and repairing mucosal epithelium and they also adjust to a diverse environment created by microorganisms due to their properties of cellular plasticity as presented by Es et al.3
The underlying mechanisms of SC regeneration in response to injury are mostly unclear, although cytokine STAT5 seems to have a functional role in this process. Depletion of STAT5 leads to reduced proliferation of SC, whereas overexpression has the opposite effect.4 Further studies will reveal the active role of SC in the understanding of epithelial homeostasis in the intestine.
INTRODUCTION OF THE ROLE OF SC AS THERAPEUTIC AGENTS TO IBD
It is well established that IBD, a collective term for the chronic inflammatory conditions UC and Crohn’s disease (CD), can affect the sigmoid colon and has various immunologic and pathogenic features that motivate the prospective for more innovative therapies in the coming years.
UC is a chronic IBD characterised by ulcers of the distal sigmoid and rectum leading to diarrhoea, haematochezia, intense abdominal pain, and GI bleeding. UC signifies a defective autoimmune response leading to the excessive inflammation of the GI mucosa due to dysregulated cytokine production by CD4+ T cells and dendritic cells (DC), coupled with a loss of immunotolerance due to low numbers of T-regulatory lymphocytes (Treg).5 Activation of IFNγ and TNFα promotes proliferation of CD4+ Th1 cells, and without Treg to counteract this activity, damage to the epithelial mucosa lining the GI tract will occur (Figure 1).5 While there is no definitive cure for UC, it has been shown in recent years that therapeutic measures to reduce the activation of these CD4+ Th1 cells through a reset of the immune system can lead to cessation of IBD symptomatology.5
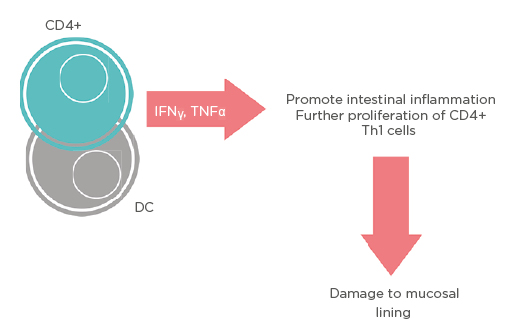
Figure 1: Schematic representation of different pathways that mesenchymal stem cells use to upregulate T-regulatory lymphocytes.
CD: cluster of differentiation; DC: dendritic cells.
Conventional treatments, such as surgical operations or drug administration, have been used over the years, with the risk of colorectal cancer (CRC) development remaining high. Patients with chronic severe and protracted UC present an increased risk of CRC, which is approximately 5%.6
The effects of a variety of anti-inflammatory agents on the progression of UC have been closely studied. Corticosteroids (CS) were the first anti-inflammatory measures that demonstrated marked success in the treatment of UC, but subsequent studies have proven an association between long-term CS therapy and complications, such as deep vein thrombosis, osteoporosis, and infection, to name a few.7 For these reasons, CS are not recommended for prolonged use and are best utilised to achieve only the initial immunosuppression necessary to promote symptom regression.
Researchers have seen moderate success with the utilisation of TNFα inhibitors in attempt to prevent hyperactivity of proinflammatory cytokines and sustain symptom remission, but this method has shown decreased efficacy in immunocompromised individuals due to complications that arise from unrelated infections and exacerbation of GI bleeding.5 Other anti-inflammatory drugs, such as JAK inhibitors, anti-integrin agents, and thiopurines have been studied for use as well, but each of these therapies have still carried substantial infection risk and have not proven to be superior in long-term maintenance of UC.8 While each of these advancements have largely proven to be beneficial in improving the prognosis of UC, the immunosuppressive nature of these agents is an inherent overlying complication to consider. With this in mind, a novel approach to treatment of UC as well as other IBD conditions has been found to be through SC transplantation, and this has been attempted in a few different approaches.
Recent published articles implicate many different types of SC in the treatment of IBD, which are HSC, bone marrow stem cells (BMSC), MSC, and ISC.9 The aim of these SC-based interventions is to reset the defective immune system by regenerating immune cells that will improve overall functionality. The alternative immunological reactions seen with anti-inflammatory drugs are not seen with SC, suggesting them to be a more effective therapy.
Researchers first saw success with the transplantation of HSC, but preliminary trials have proven that the association between this therapy and adverse events suffered after transplantation is too strong to justify further use.10 Autologous BMSC, which can aid in replenishing functional adaptive immunity that is typically lost in cases of IBD, were determined to be a much safer alternative.10 Table 1 presents some of the work that has been done regarding SC-based therapy in UC.
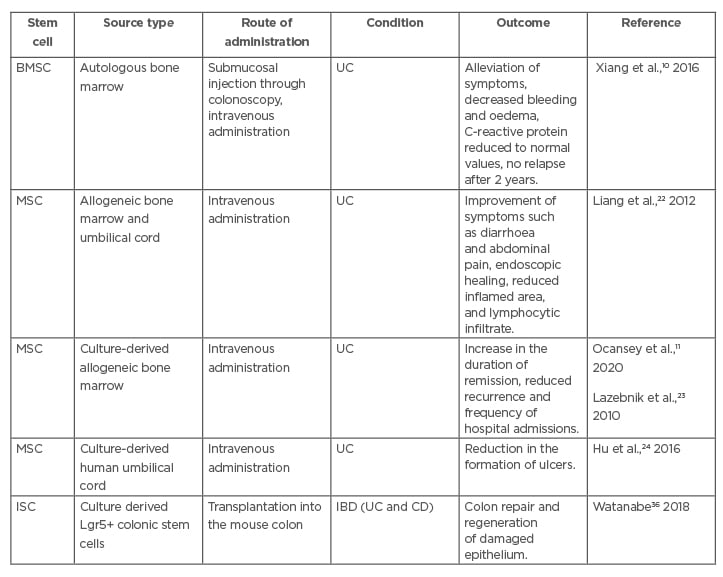
Table 1: Summarised data of some recent experimental studies about the applications of stem cells in ulcerative colitis.
BMSC: bone marrow stem cells; CD: Crohn’s disease; IBD: inflammatory bowel disease; ISC: intestinal stem cell; MSC: mesenchymal stem cell; UC: ulcerative colitis.
The application of BMSC is limited due to their painful and invasive method of sampling and the possible side effects of cyclophosphamide administered for transplantation purposes.11 Furthermore, the effects from single BMSC treatment have proven to be transient, and this has since led researchers to instead investigate the use of autologous MSC and ISC therapy.10,11 More promising data have been released so far about the therapeutic applications of MSC.
THE ROLE OF MSC AS THERAPEUTIC AGENTS IN UC
The idea of designing innovative and more promising biological therapeutic methods is well depicted in the attempts of using human MSC to treat IBD. These SC are non-haematopoietic SC derived from bone marrow, umbilical cord blood, or adipose tissue. Evidenced by several studies, it is well known that they play an important role in sites of inflammation and tissue injury due to their ability to migrate to these areas. Inflammatory tissue damage and loss of regulation of the chronic immune responses are the main features in IBD pathogenesis and MSC target them by using various molecular and cellular mechanisms.12-16
Both innate and adaptive immune responses are involved. Innate immune cells, such as antigen-presenting DC, macrophages, and natural killer cells, play major roles in the initiation of the inflammatory process in IBD. MSC react to inflammatory signals by secreting particles that suppress inflammation and proliferation of DC and natural killer cells.12,13 Moreover, it has been shown that MSC secrete cytokines that can restore a balance between M1 and M2 macrophages via inducing a switch of the M1 inflammatory phenotype to the M2 anti-inflammatory/healing phenotype.14 Experimental research has documented that MSC are important immunoregulators, enhancing the production of a variety of anti-inflammatory cytokines and growth factors, such as TGFβ, indoleamine-pyrrole 2,3-dioxygenase, prostaglandin E, IL-4, IL-10, IL-11, and IL-13, and inhibiting other proinflammatory agents, such as IL-6, IL-12, IL-23, and IL-21. 15,16
Several studies found that MSC in vitro express low levels of MHC Class I and no MHC Class II molecules. As a result, they do not elicit a T lymphocyte rejection response in cases of transplantation. The privilege of low immunogenicity of MSC is essential to overcome the barriers of the adaptive immunity, allowing their application across syngeneic and allogeneic immune systems.17
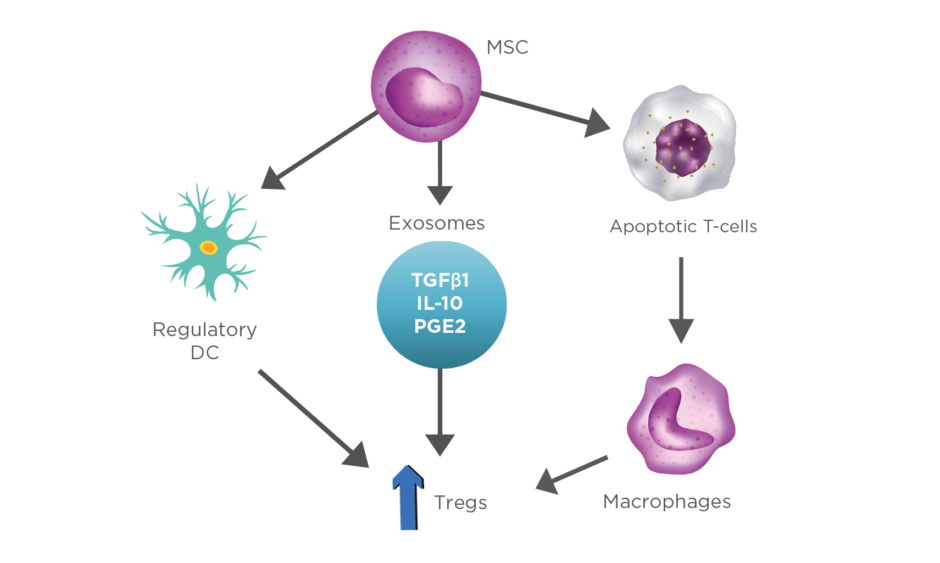
Furthermore, the adaptive immune responses are modulated by the upregulation of Tregs through various cellular mechanisms. Firstly, there has been a growing attention in the experiments where MSC drive DC to differentiate into regulatory cells, triggering the production of Tregs, which leads to a more enhanced suppression of the already established inflammation.18 Secondly, increased expression of Tregs is also presented by the secretion of exosomes and other soluble factors (TGFβ1, IL-10, prostaglandin E2) released from MSC.11,19 Lastly, MSC interfere with the dysregulation of the balance that Bax protein (proapoptotic) and Bcl-2 protein (antiapoptotic) have, resulting in enhanced T-cell apoptosis. Apoptotic T cells induce macrophages to produce high levels of TGFβ, which upregulate Tregs, thus suppressing the immunologic response (Figure 2).20,21
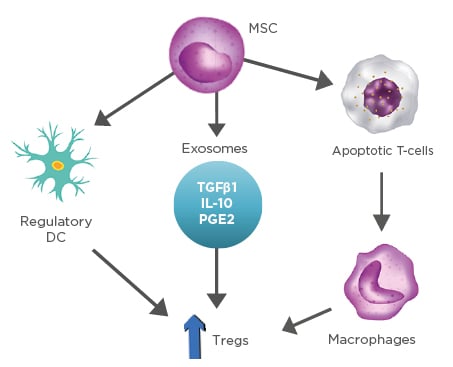
Figure 2: Representation of stem cells and their ability to modulate inflammation through production of various cytokines.
DC: dendritic cells; MSC: mesenchymal stem cells; PGE2: prostaglandin E2; Tregs: T-regulatory lymphocytes.
THERAPEUTIC APPLICATIONS OF MSC IN IBD SUMMARISED BY RECENT CLINICAL DATA
The immunomodulatory functions of MSC have led to an increased trend for their study in clinical research and therapeutic application in UC. Clinical studies on allogeneic MSC, from umbilical cord or bone marrow, reported a better clinical outcome in the disease prognosis.22 In one of these trials analysed by Lazebnik et al.,23 bone marrow-derived allogeneic MSC administered intravenously in UC patients showed a significant increase in the duration of remission, as well as reduced recurrence and frequency of hospital admissions in these patients.11 In addition, in a nonrandomised study, umbilical cord-derived MSC given by the same route reported reduction in the formation of ulcers in 30/36 patients with UC (Table 1).24
Similar studies of MSC in IBD are in different phases of clinical trials and applied in CD with promising clinical results.25 MSC have also shown efficacy in fistula healing in cases of perianal fistulising CD, demonstrated in a study by Dige et al.,26 in which 12/21 patients experiencing complete fistula healing 6 months following initial treatment with adipose-derived MSC and an additional four patients experiencing a marked reduction of symptoms.
THERAPEUTIC APPLICATIONS OF MSC IN COLITIS-ASSOCIATED CRC SUMMARISED BY RECENT CLINICAL DATA
Exploring the immunologic applications of progenitor cells in IBD let the authors consider their questionable utility for colitis-associated CRC treatment. Some recent data have demonstrated that MSC could migrate to CRC tissue from bone marrow. Due to their distinctive ability to home into neoplasia, these cells can be used as vehicles in the tumour microenvironment to target tumour sites. Modified MSC, such as TNF-related apoptosis inducing ligand MSC, MSC delivering cluster of differentiation markers, and MSC transfected with cytosine deaminase or the symporter sodium iodide and CCL5/RANTES, have been tested so far with positive correlation with the tumour regression.27 In addition, it has been reported that bone marrow MSC are able to secrete particular cytokines limiting CRC cell growth and spread.28
The immunohistochemical marker Ki67 could be a future guide to microscopically assess the therapeutic outcome of MSC transplantation examined in CRC biopsy specimens. The antitumour effect of MSC treatment has been previously studied and correlated with reduced Ki67 index. One study by Zheng et al.29proved tumour cells treated with MSC and stained with Ki67 proliferation index presented a decreased expression of this marker.
According to the above data, it seems quite safe to presume that MSC could limit or decrease the survival of tumour cells. This fact demands systematic investigation in this field in order to enlighten the specific role of MSC as therapeutic agents to colitis-associated CRC.
While MSC have proven to be predominantly effective in mediating tumour regression due to their anti-inflammatory and immunosuppressive effects, it is necessary to consider the demonstrated instances in which they have also led to outcomes opposite of this desired effect. The exact mechanism of action of MSC upon tumour cells has yet to be fully characterised; however, recent studies have shown that MSC transplantation has the potential to cause tumour growth. This is thought to be partially attributed to the tolerogenic nature of MSC as well as their preferential migration to tumour sites, which allow for creation of an optimal environment for tumour proliferation under the right circumstances.30,31 Their origins could also play a role, as evidenced in a study from Ritter et al.,32 in which it was found that certain adipose-tissue derived MSC in particular were associated with increased proliferation of malignant tumour cells, while MSC derived from the umbilical cord suppressed this growth. Patients who received allogeneic HSC transplants followed for more than two decades post-transplant showed an increased risk for secondary malignancies.33
Additionally, MSC are capable of producing vascular endothelial growth factor, which plays a key role in tumour development by inducing angiogenesis, with help from TNFα and IFNγ.34
In a study by Fu et al.,35 cancer cell lines were analysed according to their malignant phenotype, which was characterised by the proliferation rate, resistance to apoptosis, metastatic potential, and the tumorigenesis of these cells. Introductory research based on the co-culture of MSC and cancer cells suggests that these tumour-promoting effects of MSC can be exacerbated depending upon the metastatic abilities that the tumour cells possess. This indicates that it could be beneficial to first closely analyse the lineage of the existing cancerous cells in a patient before deciding to implement this therapeutic measure. MSC have been found to exert minimal influence in worsening the tumorigenic qualities of cancer cells that are already highly invasive and proliferative, in contrast to their effect on less proliferative cancer cells, in which their presence is more likely to induce tumour growth.35
Further research is necessary to better understand the discrepancies associated with MSC and their role in tumorigenesis.
THE ROLE OF ISC AS THERAPEUTIC AGENTS TO IBD AND CRC
ISC are another source that can be used for treatment. Results from preliminary studies of these therapies have proven to be promising new approaches in the treatment of UC.
Autologous transplantation of ISC has been described by Watanabe et al.36 as a safe and simple therapeutic option for patients with serious GI epithelial injuries (Table 1). Researchers anticipate transplantation of new ISC to be effective in reducing the cancer risk associated with colitis, since they are capable of replacing old ISC that often accumulate cancer-initiating mutations.37 However, further research is necessary to confirm this hypothesis and determine whether autologous ISC from IBD patients versus healthy donor ISC will have the same results after transplantation. Studies have shown that organoids extracted from CD patients have displayed the same growth capabilities as organoids from healthy individuals, which makes ISC therapy a highly positive prospect for future implication in IBD management.37 It will also be necessary to confirm that the presence of ISC does not function to aid in the progression of malignancy as is occasionally seen with MSC.
In the contest of individualised therapy in CRC patients, in vitro disease models have emerged. Samples from CRC patients establishing colon cancer organoids have contributed to the investigation of mechanisms regarding molecular markers that could contribute to therapeutic options in colon cancer.38 Biobanks of cancer organoids as well as pharmacological and genetic profiles are emerging as powerful in vitro disease models. Molecular analyses and gene profiles analysed in ex vivo ISC could help in the stratification of patients with the highest risk of developing CRC. A strong marker for ISC identified in human and mouse models from patients with IBD is a Wnt-independent cell-adhesion glycoprotein called olfactomedin 4. On the other hand, ISC from CRC patients overexpress achaete-scute-like2, a Wnt-dependent basic helix-loop-helix transcription factor.39
Vitamin D deficiency is known to be associated with IBD and CRC development, and recent studies have proven that vitamin D receptor is expressed in vivo in concurrence with the crypt SC marker LGR5.40 This discovery is significant because it demonstrated that calcitriol, a vitamin D metabolite, plays an important role in mediating ISC homeostasis. In one recent study, normal and tumour colon organoids from fresh human tissues were used to analyse the effect of calcitriol. Calcitriol inhibited the proliferation of both normal and cancer SC while simultaneously inducing cancer SC differentiation, making vitamin D receptor agonists an attractive therapeutic agent to combat CRC progression.40
CONCLUSIONS
Clinical and experimental data have shown remarkable results about the therapeutic role of SC in UC. More clinical trials are mandatory to demonstrate the efficacy of such therapies based on the cell origin, dosage, and route of administration. The establishment of reliable assessment criteria is necessary to measure the pathological and clinical effects of SC. Since many diseases of the sigmoid colon are based on chronic inflammation, further studies on the immune system could provide a more rational approach. Colitis-associated CRC and the application of SC therapies remain a challenge and an open field for more detailed research.
Altogether, right now, SC therapies constitute a novel option for treatment approach for UC and a potential new therapeutic option for other inflammatory-based sigmoid colon diseases and cancer. Future research will reveal the answers for a better orientation and the know-how to deal with therapies based on SC applications.