Abstract
Sleeve gastrectomy (SG) has been recognised as an effective procedure for the treatment of morbid obesity and associated comorbidities; however, the shortcomings of SG, such as staple line leak, haemorrhage, vomiting, and weight regain, have also been well-reported. An underestimated adverse effect of SG is nutritional deficiency (ND). While ND is a well-known complication of malabsorptive bariatric procedures, it can still occur after restrictive operations, including SG, yet its incidence and mechanism are still unclear. In an attempt to learn about the incidence and type of ND after SG we performed an organised literature search of electronic databases searching for articles that assessed the incidence and type of ND after SG. The median incidence of iron and zinc deficiency after SG was 8.8% and 18.8%, respectively. The majority of patients already had vitamin D deficiency preoperatively, with a median of 35.5% of patients still demonstrating vitamin D deficiency postoperatively. Comparing ND before and after SG, the incidence of iron and vitamin D deficiency declined postoperatively; in contrast, there was a tangible increase in the incidence of vitamin B1, B6, B12, and calcium deficiency. Vitamin B1 and B12 deficiencies were recorded in a median of 10.0% and 11.7% of patients, respectively, and were associated with neurologic manifestations in <1% of patients. Prevention of ND after SG requires proper recognition and correction of preoperative ND with immediate supplementation of trace elements and vitamins postoperatively, in addition to long follow-up.
INTRODUCTION
Morbid obesity represents a substantial health crisis across the world with a rapidly increasing prevalence.1 While lifestyle-altering measures, exercise programmes, and diet regimens manage to reduce excess body weight in some patients, bariatric surgery remains the ultimate treatment of choice for many patients who fail conservative measures. Bariatric procedures have achieved excellent results with regard to weight loss and improvement in comorbidities. However, various complications of bariatric procedures have been recognised, including anastomotic leakage, stenosis, bleeding, weight regain, and nutritional deficiency (ND).2
ND is a predictable complication after Roux-en-Y gastric bypass (RYGB) owing to the malabsorptive nature of the procedure.3 Other restrictive procedures, including sleeve gastrectomy (SG), are also associated with ND. The prevalence, type, and mechanism of ND after SG are, however, not clearly understood.4 This review aims to assess the magnitude of ND after SG and to explore the available preventative measures and treatment options.
SEARCH STRATEGY
A computerised, organised search of the English language scientific literature was conducted using the PubMed/Medline and Scopus databases. Studies reporting ND after SG performed on adult or adolescent patients with morbid obesity were included. The following keywords were used in the search process: “morbid obesity”, “obesity”, “sleeve gastrectomy”, “gastrectomy”, “restrictive”, “bariatric”, “nutritional deficiency”, “nutrient deficiency”, “micronutrient”, “vitamin B”, “vitamin D”, “folic acid”, “ferritin”, “calcium”, “iron”, and “magnesium”. The relevant articles and their list of references were screened for the type and incidence of ND after SG. After an initial screening of 332 articles and exclusion of irrelevant articles, editorials, and letters to the editors, 15 articles were considered eligible and were included in the review for analysis of the incidence of ND after SG (Table 1).
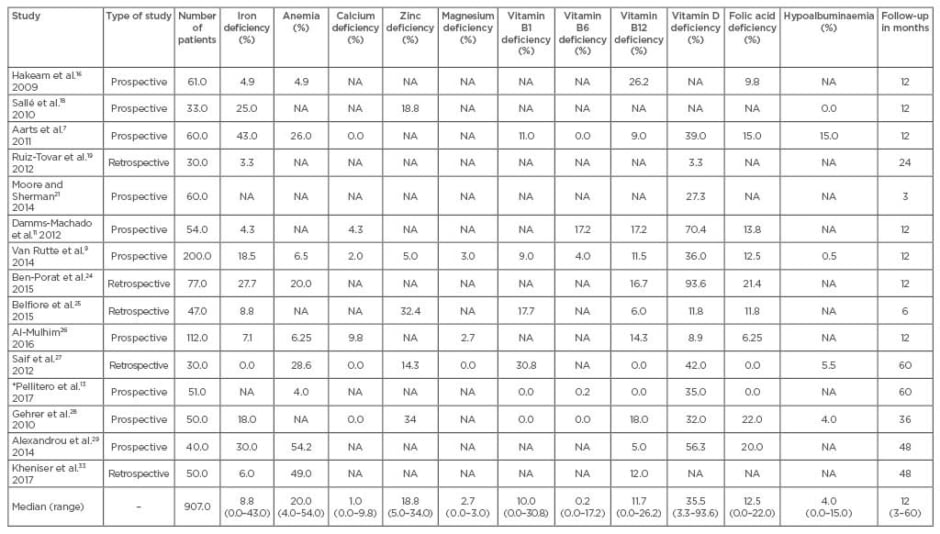
Table 1: Incidence of nutritional deficiency after sleeve gastrectomy.
*Hypocupraemia increased from 0.5% to 9.8%.
NA: not available.
AETIOLOGY OF NUTRITIONAL DEFICIENCY AFTER SLEEVE GASTRECTOMY
Several nutritional consequences of SG have been recorded, including fluid deficits, vitamin and mineral deficiencies, iron deficiency anaemia, and metabolic bone disease.5 The overall incidence of ND after SG is estimated to be 2.6%, according to a recent systematic review.2
The mechanism for ND after SG is multifactorial; it has been postulated that a pre-existing ND is already present in many patients preoperatively.6 The unhealthy eating behaviour of patients with morbid obesity usually deprives these patients of essential vitamins and minerals. Pre-existing ND, if not corrected preoperatively, will continue postoperatively with the potential for further deterioration in micronutrient levels.7-9
Resection of the gastric fundus in SG can reduce the absorption of certain micronutrients, including iron, folic acid, and vitamin B12, akin to what occurs after partial gastrectomy for peptic ulcer disease.10 In addition, the caloric restriction pattern of SG potentially contributes to folic acid, vitamin B1 and B6 deficiency, and hypocupraemia. Since hydrochloric acid is essential for iron absorption, and certain chelators such as ascorbic acid, sugars, and amino acids require acid pH to combine with soluble ferric iron to maintain it in soluble form at neutral or slightly alkaline pH, the reduced gastric acid secretion associated with SG impairs iron absorption and results in iron deficiency anaemia.11,12 The hypoacidity of the remaining gastric sleeve also impairs the absorption of copper, resulting in haematologic and neurologic abnormalities. Another reason for vitamin B12 deficiency could be the reduced consumption of vitamin B12-containing food, particularly red meat, in addition to the diminished production of the intrinsic factor responsible for the bioavailability and absorption of vitamin B12.13
It is also important to note that other gastrointestinal disorders, including coeliac disease, Helicobacter pylori infection, and atrophic gastritis, may contribute to ND after SG. In particular, H. pylori can negatively impact the absorption of iron and vitamin B12, contributing to further deficiency of these nutrients after SG. Therefore, early diagnosis and eradication of H. pylori before SG can help reduce iron and vitamin B12 deficiency postoperatively.14
The type of patient can also influence the incidence of ND after SG, particularly vitamin D and calcium deficiency. It has been demonstrated that vitamin D and calcium metabolism is impaired in postmenopausal women, hence performing SG on this category of patients may lead to further deficiency of vitamin D and calcium and may warrant the administration of higher prophylactic doses of these elements postoperatively.15
LITERATURE REVIEW
Iron Metabolism
The impact of SG on iron metabolism was reported in 2009 by Hakeam et al.16 who published information about 61 patients who underwent laparoscopic SG. Serum haemoglobin and iron indices, such as serum iron, transferrin saturation, ferritin, and soluble transferrin receptor (sTf-R), were measured preoperatively and at 6 and 12 months after SG. Iron deficiency and anaemia were recorded in three (4.9%) patients at the 12-month follow-up. Different patterns of iron deficiency were recognised; the first had an abnormally elevated sTf-R level, the second had low serum iron and transferrin saturation with high sTf-R, while the third had low levels of both ferritin and iron with reduced transferrin saturation. Furthermore, a significant increase in the incidence of vitamin B12 deficiency was noted (8.1% increased to 26.2% postoperatively), and a similar increase in folic acid deficiency was also observed (0% increased to 9.8% postoperatively).
Iron deficiency after SG can be explained partially by a marked reduction in iron absorption after SG. According to Ruz et al.,17 a significant decrease in the absorption of haem-iron (23.9% reduced to 6.2%) and non-haem-iron (11.1% reduced to 4.7%) was recorded 12 months after bariatric surgery, including SG. This observation indicates a need for an effective increase in iron supplementation after SG to prevent iron-status impairment.
Zinc Metabolism
Sallé et al.18 concluded that zinc deficiency is a frequent, yet underestimated, problem after bariatric surgery. Zinc deficiency was found to be less frequent after SG compared to RYGB and biliopancreatic diversion. Among 33 patients who underwent SG, 18.8% suffered zinc deficiency 1 year postoperatively compared to 6.5% before surgery. The mean serum level of zinc showed a non-significant decrease on follow-up. The incidence of patients with iron deficiency increased from 21.7% preoperatively to 25.0% at 1 year after SG; the authors attributed the increased deficiency to either inadequate protein intake, a defect in the compensatory mechanism of the gut and liver, or insufficient intake of dietary zinc.
Aarts et al.7 emphasised that patients after SG are at serious risk of developing ND because of inadequate intake and uptake of micronutrients and nutrients. Among 60 patients, 43% developed iron deficiency, 39% suffered from vitamin D deficiency, 26% developed anaemia, 15% had folic acid deficiency, 15% developed hypoalbuminaemia, and 9% had vitamin B12 deficiency.
Calcium and Vitamin D Metabolism
The mid-term effect of SG on calcium and vitamin D metabolism was investigated by Ruiz-Tovar et al.19 on 30 females. The incidence of vitamin D deficiency declined from 96.7% preoperatively to 3.3% at 1-year postoperative follow-up. One patient had hypoalbuminaemia and another had folic acid deficiency before SG; both patients had normal serum albumin and folic acid levels postoperatively. Serum levels of vitamin B12, vitamin D, folic acid, and iron considerably increased at 24 months postoperatively compared to a modest increase in serum levels of albumin, zinc, and calcium. This study was the first of its kind to report increased vitamin D levels after bariatric surgery, namely SG. A plausible explanation for this phenomenon was that the study was conducted in Spain, which has a higher sunlight exposure than North America and northern Europe where the previous studies were undertaken. According to the authors, patients were encouraged to practice outdoor activities to enhance physical performance that helped increase exposure to sunlight.
Parallel to their previous study, Ruiz-Tovar et al.20 investigated changes in bone mineral density (BMD) at 1 and 2 years post SG. A statistically significant increase in the BMD values of the spine was noted at both follow-up points (5.7% increase at 1 year and 7.9% increase at 2 years). Furthermore, a direct correlation was observed between BMD and vitamin D increase. Moore and Sherman21 suggested that vitamin D deficiency after bariatric surgery, including SG, is caused by reduced food intake. The authors advocated a daily supplementation with 2,000 IU of vitamin D3 and 1,500 mg calcium citrate; they found this protocol successful in reducing the incidence of vitamin D deficiency from 54.5% preoperatively to 27.4% 3 months after SG.
Lanzarini et al.22 advocated that “Patients undergoing bariatric surgery should receive high-dose vitamin D supplementation independently of the surgical technique”. Patients who underwent SG or RYGB were administered 400 IU/day of 25(OH)D with additional supplementation of 16,000 IU of vitamin D3 every 2 weeks if 25(OH)D serum levels were <30 ng/mL. Normal vitamin D levels were recorded in 69% of patients that received high-dose vitamin D supplementation, compared to 48.3% in the group that received the regular dose of the vitamin. This recommendation was supported by the conclusion of a randomised trial23 that a supplementation of 80 μg/day of oily vitamin D3 effectively prevents vitamin D deficiency as well as to treat pre-existing deficiencies after SG.
Comparing Nutritional Deficiency Before and After Sleeve Gastrectomy
Damms-Machado et al.11 compared pre and postoperative nutritional status after SG. At 1 year postoperatively, the incidence of vitamin D and iron deficiency declined from 83.0% to 70.4%, and from 29.0% to 4.3%, respectively. Conversely, the incidence of vitamin B6, vitamin B12, and folic acid deficiency increased from 11.1% to 17.2%, 9.3% to 17.2%, and 5.5% to 13.8%, respectively. While none of the patients had calcium deficiency preoperatively, 4.3% of patients had low serum calcium levels postoperatively.
Van Rutte et al.9 also assessed ND before and 1 year after SG, and noted that the incidence of anaemia increased from 5.0% to 6.5%, whereas iron deficiency declined from 38.0% to 18.5%. Calcium deficiency increased from 0.5% to 2.0%, and magnesium deficiency from 2.0% to 3.0%. The incidence of phosphate deficiency decreased from 14.0% to 3.5%, whereas zinc deficiency increased from 0.0% to 5.0%. Folate deficiency declined from 24.0% to 12.5% and vitamin D from 81.0% to 36.1%, while vitamin B1 increased from 5.5% to 9.0% and vitamin B6 from 3.0% to 4.0%. The incidence of vitamin B12 deficiency remained the same both pre and postoperatively, equal to 11.5%.
Conversely, Ben-Porat et al.24 found the effect of SG on ND at 1 year postoperatively to be modest. The incidence of anaemia, low ferritin levels, and vitamin B12 deficiency increased from 11.4%, 8.3%, and 11.7% before surgery to 20.0%, 11.1%, and 16.7% postoperatively, respectively. Conversely, iron deficiency decreased from 40.4% to 27.7%, folate deficiency from 40.5% to 21.4%, and vitamin D deficiency from 97.9% to 93.6%. Preoperative deficiencies of haemoglobin, folate, and B12 proved to be significant predictors for deficiencies 1 year after SG. The study concluded that proper recognition of preoperative ND alongside tailoring a specific supplemental programme for each individual should prevent postoperative ND.
Belfiore et al.25 used bioelectrical impedance analysis to assess changes in body composition after SG. The energy intake exhibited a remarkable decrease after SG that was associated with marked changes in body composition demonstrated as a decrease in fat-free mass and fat mass 3 months postoperatively. With further follow-up, the fat-free mass loss slowed down whereas the decrease in fat mass continued for 6 months postoperatively. Iron deficiency decreased from 14.9% to 8.8%, vitamin B12 from 10.7% to 6.0%, folate deficiency from 19.1% to 11.8%, and vitamin D deficiency from 31.9% to 11.8%. In contrast, vitamin B1 deficiency increased from 0.0% to 17.7%, and zinc from 4.3% to 32.4%.
Al-Mulhim26 described vitamin and ND after SG as a common phenomenon. The incidence of anaemia declined from 24.0% to 6.25%, iron deficiency from 11.6% to 7.1%, vitamin D deficiency from 60.0% to 8.9%, and magnesium from 6.2% to 2.7%. In contrast, the percent of patients with folate, vitamin B12, and calcium deficiency increased from 0.9%, 1.8%, and 0.0% to 6.25%, 14.3%, and 9.8%, respectively. The author devised a set of important recommendations to prevent ND after SG, including correction of preoperative ND, sufficient supplementation immediately after SG, and long follow-up.
Long-Term Effects of Sleeve Gastrectomy
The long-term influence of SG on nutrient status was studied by Saif et al.27 in 82 patients with morbid obesity. At 5-year follow-up, vitamin D deficiency, vitamin B1 deficiency, low serum haemoglobin levels, hypoalbuminaemia, and zinc deficiency were recorded in 42.0%, 30.8%, 28.6%, 5.5%, and 14.3% of patients, respectively. No patients were recorded to have either iron, calcium, or magnesium deficiency at 5-year follow-up. Roughly 43.0% of patients reported taking supplements 3 years after SG and this percentage increased to 63.3% at Year 5.
Pellitero et al.13 evaluated long-term status of vitamins and micronutrients after SG; 176 patients were prospectively followed for up to 5 years after surgery. The incidence of anaemia declined from 23.9% to 4.0%, vitamin B12 deficiency from 6.9% to 0.0%, folic acid deficiency from 6.5% to 0.0%, vitamin D deficiency from 73.0% to 35.0%, vitamin B1 deficiency from 3.4% to 0.0%, and vitamin B6 deficiency from 12.0% to 0.2%. Conversely, hypocupraemia increased from 0.5% preoperatively to 9.8% 5 years postoperatively, whereas none of the patients had selenium deficiency neither before nor after SG.
Comparing Sleeve Gastrectomy and Roux-en-Y Gastric Bypass Regarding Postoperative Nutritional Deficiency
Gehrer et al.28 compared ND after SG with RYGB in 136 patients. The incidence of ND at 1 year postoperatively was generally less after SG than RYGB (34% versus 37% for zinc; 18% versus 28% for iron; 4% versus 8% for albumin; 32% versus 52% for vitamin D; and 18% versus 58% for vitamin B12) except for folate deficiency which was higher after SG than RYGB (22% versus 12%). Patients in both groups exhibited normal serum levels of calcium, vitamin B1, and vitamin B6. ND after SG was easily treated with nutritional supplementation in the form of intramuscular cyanocobalamin, oral folic acid therapy, intravenous therapy with iron(III)-hydroxide sucrose or oral administration of iron(II)-glycine sulphate, oral zinc gluconate 30 mg therapy, and oily suspension of cholecalciferol (300,000 IE).
Alexandrou et al.29 compared the long-term influence of SG and RYGB on the micronutrient levels and found both procedures to be associated with significant ND. At 4-year follow-up, the incidence of vitamin B12 deficiency, anaemia, and iron deficiency was lower after SG compared to RYGB (5.0% versus 42.1%; 54.2% versus 64.3%; and 30.0% versus 36.4%, respectively), whereas folate and vitamin D deficiencies were more frequently detected after SG than RYGB (20.0% versus 18.4%, and 56.3% versus 39.6%, respectively). It is important to note that there were no significant differences in postoperative ND among the two procedures with exception to vitamin B12 deficiency.
A meta-analysis of nine studies that assessed ND after SG and RYGB30 concluded that both operations had similar odds for developing anaemia and iron deficiency postoperatively; however, RYGB had higher odds for developing postoperative vitamin B12 deficiency than SG (odds ratio: 3.5; p=0.001). Since vitamin B12 deficiency is more frequent after RYGB, Majumder31 proposed that while patients undergoing SG need vitamin B12 supplementation postoperatively, they may be maintained on a lower daily dose of vitamin B12, lower than the regular dose prescribed for patients with RYGB.
According to Brandão et al.32 both RYGB and SG had no influence on the serum level of vitamin A and visual function postoperatively. Conversely, Kheniser et al.33 demonstrated that a significantly higher number of SG patients developed postoperative anaemia compared to RYGB patients (49% versus 23%; p=0.01). The percent of patients who had deficiencies in the mean corpuscular volume, haematocrit, transferrin, red blood cell folate level, iron, and vitamin B12 were higher after SG than RYGB.
Neurologic Complications
Micronutrient deficiency after SG may lead to neurologic manifestations as seen in vitamin B1 (thiamine) deficiency, which results in Wernicke’s encephalopathy (WE). A systematic literature review34 included 13 reports describing the occurrence of WE after SG with the majority of patients being female. Thiamine deficiency was attributed to gastric wall oedema and non-dietary compliance. The diagnostic triad of WE include ocular impairment and nystagmus, cerebellar dysfunction, and confusion in addition to severe polyneuropathy in some cases. WE usually responds to intensive therapy of thiamine with complete resolution of symptoms occurring within a few months.
Vitamin B1 deficiency can also result in beriberi, a condition that is associated with severe peripheral polyneuropathy characterised by sensory and motor losses. Durán et al.35 reported a case of a young female who developed progressive paraesthesia and intense pain and loss of strength in her lower limbs disabling the patient from walking 6 weeks after SG. The patient showed a slow but steady response to parenteral thiamine therapy. Three months later, the patient was discharged on vitamin supplementation and physiotherapy.
Another serious neurologic consequence of vitamin deficiency was reported by Sawicka-Pierko et al.36 who presented the case of a middle-aged female who developed optic neuropathy 10 months after SG. The patient presented with bilateral decrease of visual acuity and a bilateral loss of visual field. A marked decrease in serum level of vitamin B12 was noted. Intramuscular injection of vitamin B12 managed to resolve the visual symptoms and tripled the serum vitamin B12 level within 1 week of therapy. The authors recommend that all patients receive long-term ophthalmological follow-up after SG.
Punchai et al.37 reported neurologic manifestations including WE, paraesthesia, muscle weakness, abnormal gait, and polyneuropathy secondary to vitamin B deficiencies in 0.7% of patients after a median duration of 12 months. After nutritional supplementation, resolution of neurologic symptoms occurred in 85% of patients yet WE was not fully reversible.
LIMITATIONS
This review is limited by the relatively small number of studies included (Table 1). Furthermore, the majority of the studies measured the micronutrient level in the serum or plasma, not the whole blood, which may affect the reliability of these measurements; plasma contains coagulant factors that may interfere with accurate assessment of trace elements. In addition, most of the studies focussed on the changes in biochemical parameters after SG without elaborating on the clinical impact of these changes on the outcome of patients.
CONCLUSION
The median incidence of iron and zinc deficiency after SG was around 9% and 20%, respectively. The majority of patients already had vitamin D deficiency preoperatively due to sequestration of vitamin D in fat reserves; a median of 35.5% of patients still suffered vitamin D deficiency after postoperatively. Comparing ND before and after SG, the incidence of iron and vitamin D deficiency seemed to decline postoperatively. On the contrary, vitamin B1, B6, B12, and calcium deficiency showed a tangible increase. A deficiency in vitamin B1 and B12 was recorded in a median of 10.0% and 11.7% of patients, respectively, and was associated with neurologic manifestations in <1.0% of patients.








