Abstract
Fat accumulation in the pancreas, defined as fatty pancreas, is usually an incidental finding during transabdominal ultrasound examination. Fatty pancreas without any significant alcohol consumption is defined as non-alcoholic fatty pancreas disease. Even though its clinical impact is still largely unknown, hypothetically the disease progression could lead to chronic pancreatitis and possibly pancreatic cancer development. Recently, metabolic problems such as diabetes, central obesity, fatty liver, and dyslipidaemia have been considered important risk factors related to non-alcoholic fatty pancreas disease and pancreatic cancer; however, the exact mechanism is not yet fully understood. Early detection and screening for pancreatic cancer in clinical practice is troublesome because of the non-specific symptoms, anatomical location, accuracy of biomarkers in clinical practice, and high risk of radiation and contrast agent exposure from imaging study. Endoscopic ultrasound is still considered the best method for pancreas evaluation and for the screening and diagnosis of pancreatic cancer. However, there is still much debate regarding its cost, availability, and the training experience of the operator.
INTRODUCTION
Non-alcoholic fatty pancreas disease (NAFPD) is a new clinical entity where there is evidence of excessive pancreatic fat accumulation in patients without any significant alcohol consumption.1,2 The impact of this condition is still largely unknown even though it has been postulated that fatty pancreas may lead to chronic pancreatitis and is a possible cause of pancreatic cancer. Pancreatic cancer is the most lethal cancer in the world and early detection is still difficult due to its location and non-specific symptoms.3
Endoscopic ultrasound (EUS) examination is the most sensitive tool for examining the pancreas in the era of modern imaging development; however, the availability, cost, and training are still debatable, especially in most developing Asian countries.4,5 In this review, the new paradigm of NAFPD, risk factors, its clinical impact on pancreatic cancer development, and screening modalities for early detection are discussed.
NON-ALCOHOLIC FATTY PANCREAS DISEASE AND METABOLIC RISK FACTORS
Fatty infiltration in the pancreas or fatty replacement in pancreas cells are considered benign conditions. This condition was first described by Schaefer6 in 1926 who performed a biometric study, and later by Ogilvie7 in 1933 who studied the pancreas through autopsies; however, the impact of this condition in clinical practice is still largely unknown.8 The prevalence of NAFPD has been reported in the USA as well as in Asian countries as ranging from 16–35%.9-12 Later, the term NAFPD was described in relation to obesity and metabolic syndrome. Free fatty acid (FFA) is key to insulin resistance pathogenesis; this well-known condition makes a significant contribution to most metabolic disorders, such as diabetes, hypertension, dyslipidaemia, and non-alcoholic fatty liver disease (NAFLD). There have been many studies showing that metabolic factors are strongly related to NAFPD.13-16 Fat accumulation in the pancreas is hypothesised to be strongly related to the increase of circulating FFA, not only from the visceral adipose tissue but also predominantly from the subcutaneous adipose tissue. That is why obesity, and especially central obesity, is thought to play the biggest role in NAFPD development and its progression.17
A strong association between the presence of NAFLD and NAFPD has been shown in some studies.18-20 This concomitant phenomenon could be as high as 50% in both Asian and Western countries, these conditions share the same possible pathogenesis. Studies in the Hong Kong Chinese population showed a significant correlation between NAFPD and NAFLD (odds ratio [OR]: 2.22; 95% confidence interval [CI]: 1.88-2.57; p<0.001).18-20 A study found that there was a direct relationship between fatty pancreas and fatty liver in central obesity.21 In patients with biopsy-proven NAFLD, the pancreatic fat was shown to correlate significantly with the severity of hepatic steatosis.21 There is a controversial study about this finding; however, the study sample was too small.22,23
Other investigations revealed that there is a strong relationship between NAFPD and diabetes, even though there is no prospective study yet to explore the clear mechanism in this condition.12,16 Wong et al.12 found that patients with NAFPD have a higher chance of developing insulin resistance. This study also showed that obesity and hypertriglyceridaemia were independent risk factors for NAFPD (OR: 1.79 and 3.16, respectively). It has been postulated that NAFPD can precede pre-diabetes and lead to diabetes development. Even though the exact mechanism is not yet clear, the damage and fat replacement at the acinar cells could further lead to β-cell dysfunction. This fat accumulation could lead to reactive oxygen species activation, increasing oxidative stress and resulting in β-cell apoptosis.24,25 Alternatively, another possible mechanism of fatty pancreas development is due to congenital syndromes, including cystic fibrosis, Shwachman–Diamond syndrome, Johanson–Blizzard syndrome, and heterozygous carboxyl-ester-lipase mutations.1
Detection of pancreatic steatosis is challenging in clinical practice, since it is usually an incidental finding during transabdominal ultrasound. The diagnosis of fatty pancreas is based on ultrasound imaging that shows diffuse hyperechoic parenchyma when compared to the kidney. Its retroperitoneal anatomical location makes the pancreas more difficult to visualise, especially in overweight or obese patients. Some imaging modalities have been used to quantify the fat content, such as magnetic resonance imaging (MRI), since this modality can identify more accurate fat infiltration. However, transabdominal ultrasound is still easier to use without any risk of radiation or contrast agent.2
PANCREATIC CANCER, RISK FACTORS, SCREENING, AND EARLY DETECTION
Pancreatic cancer is still the most lethal cancer in the world, and it has a very poor prognosis. Most patients with pancreatic cancer present at a late stage of the disease because it is still difficult to detect in the earlier stages of cancer development.26 Smoking, chronic pancreatitis, diabetes, and heavy alcohol consumption are the most common risk factors for pancreatic cancer development. However, obesity and metabolic syndrome are also considered to be important risk factors for pancreatic cancer development even though the exact mechanism requires further study. There are several pathways that explain the role of metabolic syndrome and cancer development, such as insulin-like growth factor-1 (IGF-1) pathway, hyperinsulinaemia, insulin resistance, and impact of hyperglycaemia. Evidence from colon cancer cases show a strong correlation between overexpression of IGF-1 receptors and apoptosis resistance cancer cells. In diabetes or metabolic syndrome patients, the levels of IGF-1 are decreased. Other conditions, such as insulin resistance and advanced glycation end-products due to hyperglycaemia, are also related to more advanced cancer.27,28 The phenomenon can be explained based on interaction between factors.
The pancreas is composed of endocrine and exocrine cells. Most pancreatic cancers, originate from the exocrine gland; ductal adenocarcinoma is the most common type. The acinar cells injury is initiated by fat replacement when there is high FFA released by peripheral adipose tissue. The excess fat replacement will worsen the fatty pancreas condition. High FFA, especially in obese patients, will also cause an imbalance of adipocytokines and lead to inflammation. Fatty pancreas or pancreatic steatosis has been hypothesised to have a similar mechanism with the spectrum of NAFLD. It has been postulated that oxidative stress can arise from long-standing fat accumulation, which leads to proinflammatory cytokine release. Chronic fat accumulation in the pancreas with chronic inflammation may lead to chronic pancreatitis and, possibly, to cancer development.17
Screening early pancreatic cancer is another challenge in clinical practice. Most pancreatic cancer symptoms are non-specific; the most common symptoms are dyspepsia, back pain, abdominal pain, bloating, changes in bowel habit, lethargy, and weight loss. It is important to have an accurate tool for screening and detection during the very early stages of the disease. There are biomarkers and imaging techniques that are usually used for early detection and screening. The most common marker used to detect pancreatic cancer development is CA 19-9. However, the wide range of sensitivity and specificity (68–91%) means this marker is not ideal for clinical use; additionally, high levels of CA 19-9 can also be found in several other conditions, such as cholangitis, gastrointestinal cancer, and biliary cancer, which could potentially result in misdiagnosis.29 Other biomarkers, including CEACAM1, MMP-7, TIMP-1, and MUC1, have been studied; however, even though some of these markers showed better accuracy in cancer detection when combined with the older screening mechanisms, CA 19-9 is still considered inadequate, especially due to genetic heterogeneity. Another study29 looking at a combination of CA 19-9, albumin, and IGF-1 showed that this combination could differentiate between chronic pancreatitis and pancreatic cancer with a sensitivity and specificity >90%. The major drawback is that the training set of the biomarkers may not be valid due to a higher level of collected samples. A novel biomarker study,30 based on a microRNA assay, has also been carried out.29,30 However, there is a controversial issue regarding the removal of pancreatic juice through invasive procedures, such as endoscopic retrograde cholangiopancreatography. microRNA has been used for plasma examination; however, a challenge with this detection method is ensuring diagnostic accuracy. To ensure the accuracy of this method before it is used in routine clinical examination, it should be validated in a large cohort. Imaging techniques, such as multi-detector helical computed tomography (CT) scan and MRI, have shown good diagnostic accuracy in the detection of pancreatic cancer. The sensitivity and specificity ranged from 80% to >90%.30 However, the radiation exposure and contrast agent issues mean a regular CT scan is not the preferred option for routine screening and detection. Imaging techniques such as magnetic resonance cholangiopancreatography could provide useful information about early pancreatic changes, but the patient’s co-operation during examination and the time needed for each examination makes this technique uncomfortable for most of the patients, especially elderly patients.31
ENDOSCOPIC ULTRASOUND AND ITS IMPACT IN PANCREATIC CANCER SCREENING AND SURVEILLANCE
In the diagnostic development era, endoscopic ultrasound (EUS) is known as the most sensitive method for the pancreas and biliary system. This tool has been introduced in 1980 and mostly used for diagnostic purposes only. Recently EUS has been used not only for diagnostic but also for interventional (such as fine needle aspiration [FNA]) and therapeutic purpose (such as EUS pancreatic pseudocyst drainage, EUS celiac axis block, and EUS biliary drainage).4,32
Pancreatic cancer screening and high pancreatic risk lesion surveillance has become a big topic for debate. The high-risk individuals are persons who have a strong family history of pancreatic cancer, especially in two or more first-degree relatives, other inherited conditions such as familial adenomatous polyps, HNPCC, BRCA1 carrier, hereditary pancreatitis, and cystic fibrosis. Patients with familial atypical multiple mole melanoma (FAMM) and Peutz–Jeghers syndrome also part of the high-risk individuals. The poor survival rate and the lack of significant increase in success rate in pancreatic cancer even after surgery (only 10–20% for 5-year survival rate) makes improving diagnostic efforts a vital endeavour. The small percentage increase of pancreatic cancer incidence every year results in the current screening programmes losing cost-effectiveness. Currently, however, screening in the early stages of the disease and finding the advanced stage (including involvement of lymph node and vascular invasion) remain the only hope for longer survival achievement. Detection of high-risk lesions may be more useful since surgery can be done in the very early stage of the disease. A study by Brentnall et al.33 in 14 patients showed that EUS has an important role in diagnosing some abnormalities that could not detected by CT scan or MRI. Surgery results revealed dysplasia lesions in some of the patients who were previously highlighted by EUS procedures. Another screening and surveillance study34 for pancreatic cancer detection in some family members of patients with high risk factors, such as HNPCC, FAMM, and Peutz–Jeghers syndrome, found that some of these patients’ family members suffered from pancreatic cancer.
The imaging studies, such as CT scan, MRI, and even positron emission tomography could not detect any abnormalities in these pancreatic cancer patients; however, they were detected by EUS examination.34 This issue has also been proven by some studies where EUS was performed in high-risk patient groups and when detecting high-risk lesions or possible precursor lesions in patients that underwent surgery. After surgery, significantly longer survival was achieved (disease free >5 years); however, it is important to note the small sample size used in these studies.35,36 The main problem in real-world clinical practice is that no symptoms are present in pancreatic cancer lesion <1 cm and most patients come with painless jaundice or biliary obstruction which is a sign of advance disease.37
Compared to other imaging modalities, the close range of pancreas examination from the stomach makes EUS the most accurate imaging modality (80–90%) even though it is still operator dependent (Figure 1).38 The EUS-FNA (Figure 2) technique enables ‘one-stop shopping’, where biopsy of the suspicious nodule can be performed at the same time as screening; furthermore, EUS-FNA has been proven to have high diagnostic accuracy (>90%).39 This method has overcome the old method of endoscopic retrograde cholangiopancreatography to do a biopsy or brush cytology. Another advantage of using EUS is that it can detect smaller (<2 cm) lesions more accurately than CT scan or MRI.40 A systematic review41 comparing EUS with CT scan examination in pancreatic mass detection showed the superiority of EUS over CT scan (Table 1). However, MRI can detect small cysts accurately, meaning the combination of EUS and MRI has become the main option in screening precursor lesion.40,41 Recently, most of the cystic lesions in the pancreas can be clearly evaluated by EUS examination, especially for intraductal papillary mucinous neoplasm and mucinous cystic neoplasm. The carcinoembryonic antigen from the cystic fluid can also be an important marker to predict high-risk cystic lesions. However, since most cystic lesions do not progress to malignant lesions, the routine use of EUS screening is being debated, especially regarding the cost, training to carry out the process, and the invasiveness of the procedure. Another difficult problem with EUS is diagnosing possible malignancy in patients with chronic pancreatitis, diffusely infiltrating cancer, and recent acute pancreatitis. The novel technology, EUS elastography, is a promising tool for detecting pancreatic mass more accurately than conventional EUS because it can give a better visualisation of tissue elasticity so it can target the lesion by avoiding less fibrotic areas.42,43 Transabdominal ultrasound may be more simple, comfortable, and cost-effective for most of the patients; however, the anatomical location and the patient’s preparation and condition can make the process difficult to assess.44
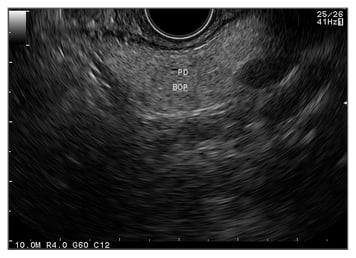
Figure 1: Endoscopic ultrasound image showing bright hyperechoic of pancreas parenchyma.
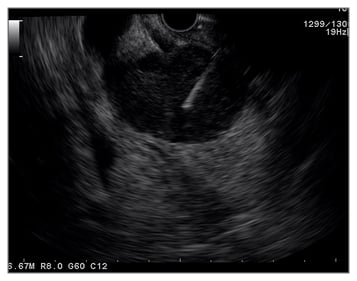
Figure 2: Endoscopic ultrasound image showing a fine needle aspiration performed in a pancreatic head mass.
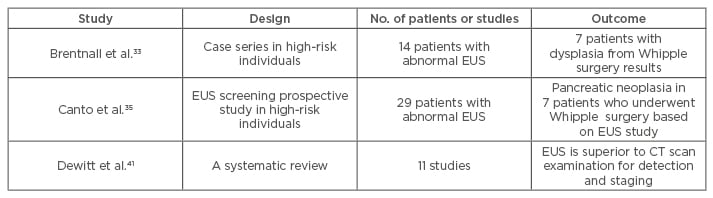
Table 1: Impact of endoscopic ultrasound study on pancreatic cancer detection.
CT: computed tomography; EUS: endoscopic ultrasound.
CONCLUSION
NAFPD is a new emerging disease that is an important risk factor for pancreatic cancer development. The role of EUS in early detection and screening surveillance has given a new insight into the field of gastroenterology. Patients with high-risk factors, such as HNPCC, FAMM, and Peutz–Jeghers syndrome, and their family members should be strongly recommended to undergo EUS screening and surveillance. Patients with metabolic risk factors, such as NAFLD, diabetes, obesity, and dyslipidaemia, might be considered for early screening examination. However, the risk-benefit ratio should always be the main consideration in clinical practice.








