Abstract
Lupus enteritis is a rare presentation of systemic lupus erythematous, clinically manifested by abdominal pain, vomiting, and diarrhoea. Proper diagnosis and treatment are essential to avoid complications, including death. Here, the authors report a case of a 52-year-old White female who presented with abdominal pain and chronic diarrhoea, with diagnostic tests compatible with lupus enteritis. Such a condition is an uncommon manifestation of systemic lupus erythematosus, an autoimmune disease that affects young females, resulting from gastrointestinal involvement by small vessel vasculitis. Early detection and proper management of lupus enteritis are essential to improve long-term survival. The present case addresses the clinical characteristics of lupus enteritis, emphasising its pathophysiology, diagnosis, and treatment.
Key Points
1. The authors report a case of lupus enteritis as the first presentation of systemic lupus erythematosus, with a difficult diagnosis, emphasising the clinical manifestations, its pathophysiology, and its importance to differential diagnosis in cases with abdominal pain and chronic diarrhoea.
2. This is a case of a 52-year-old White female with abdominal pain and chronic diarrhoea, with diagnostic tests compatible with lupus enteritis. The present case addresses this clinical condition and aims to give importance to this differential diagnosis in chronic diarrhoea, emphasising the pathophysiology, clinical picture, diagnosis, and treatment.
3. Lupus enteritis is a rare presentation of systemic lupus erythematous, and proper diagnosis and treatment are essential to avoid relevant complications, including death. Its clinical presentation is somewhat non-specific and can be confused with several chronic gastrointestinal diseases, making it a challenge even for specialised care teams in gastroenterology, as its importance to differential diagnosis in cases of chronic diarrhoea.
INTRODUCTION
Lupus enteritis is a rare and poorly understood cause of abdominal pain in patients with systemic lupus erythematosus (SLE).1 It is characterised by intestinal wall inflammation secondary to the deposition of immune complexes and complement system activation, resulting in vasculitis and ischaemic injury.2 Patients may present with abdominal pain, vomiting, diarrhoea, and fever,3 with a risk of complications such as intestinal infarction, obstruction, and perforation.1 The authors report a rare case of lupus enteritis as the first manifestation of SLE and discuss other presentations available in the literature. The study of lupus enteritis is relevant since it can be confused with conditions of significant morbidity and mortality, including mesenteric or hepatic thrombosis, intestinal obstruction and perforation, and other common causes of abdominal pain.
CASE REPORT
A 52-year-old White female with no previous comorbidities was admitted to the emergency department with severe abdominal pain in the mesogastrium, obstipation, vomiting, and abdominal distention. They had lost 15 kg in weight over the past 4 months. On physical examination, the patient was pale, with dehydrated mucous membranes, afebrile, tachycardia (heart rate: 112); however, their other vital signs stable.
The abdomen had reduced bowel sounds, pitting, was tympani, and was painful on palpation, without peritonism. Initial laboratory tests showed anaemia, lymphopenia, increased C-reactive protein, and other abnormalities as hypokalaemia. Abdominal CT (Figure 1A and B) showed small bowel distension with air-fluid levels, signs of jejunal pneumatosis, wall thickening of the small intestine and right colon (target-like appearance), free fluid in the peritoneal cavity, and portal vein gas, with no evidence of intestinal obstruction. The patient received intravenous fluids, antibiotics, gastric decompression with a nasogastric tube, and a parenteral diet.
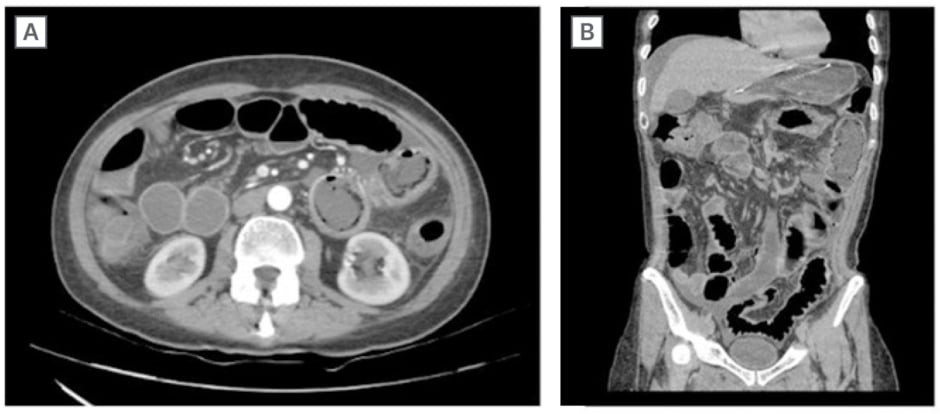
Figure 1: Axial (A) and coronal (B) CT scan of the abdomen demonstrating diffuse thickening of the intestinal wall, small intestine, and right and transverse colon, as well as distension of the intestinal loops.
Before this presentation, the patient was under medical investigation for chronic diarrhoea, which had started 4 months earlier, with three episodes a day of yellowish, large-volume stools (Bristol stool scale: 7).
During their hospital stay, the patient received a 3-day course of nitazoxanide for intestinal parasitosis, with no response. As the patient used antibiotic regimens with increased risk for Clostridium difficile proliferation, they received a 10-day course of oral vancomycin for pseudomembranous colitis, with no response. They were empirically treated for bacterial overgrowth with rifaximin 550 mg/day for 14 days, showing transient symptoms relief.
An upper digestive endoscopy raised the suspicion of duodenal atrophy; however, a colonoscopy showed flat erosions in the sigmoid and rectum, with normal terminal ileum. The anatomopathological examination of these endoscopic abnormalities was unrevealing.
The patient’s status worsened after an episode of intestinal sub-occlusion when serum tests suggested the presence of SLE: positivity for anti-nuclear antibody (1/160 with dotted nuclear pattern), thrombocytopenia, and sub nephrotic proteinuria (protein/creatinine ratio of 0.8). Lupus enteritis was confirmed after rheumatological evaluation and new tests showing low complement levels (C3 and C4) and positivity for anti-Sjögren’s-syndrome-related antigen A (Table 1).
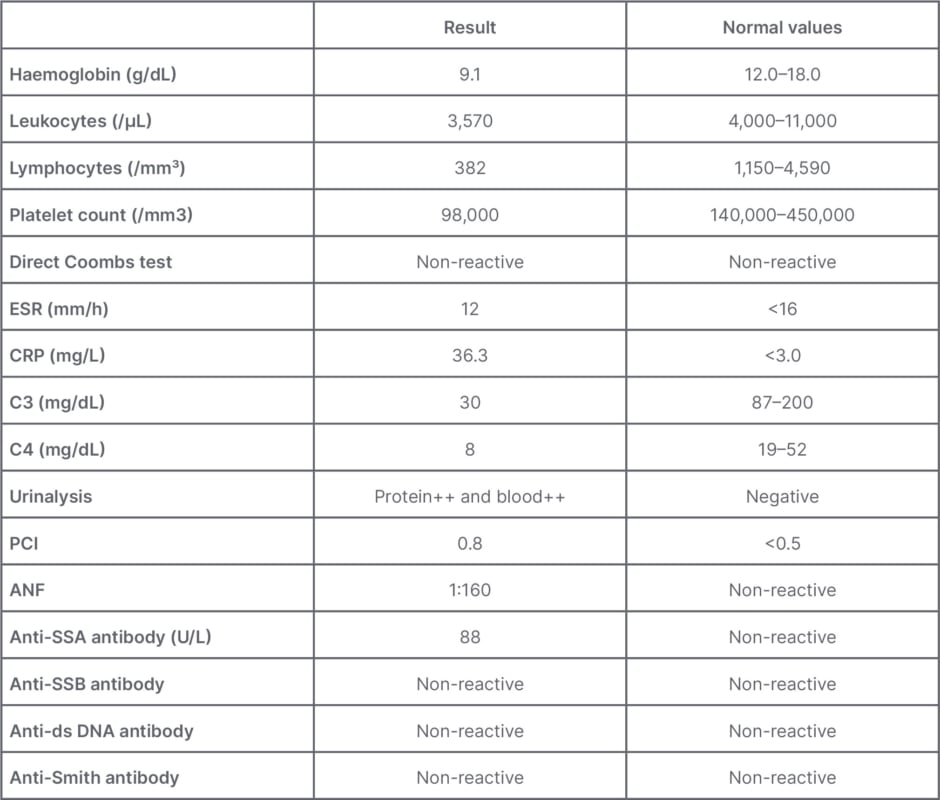
Table 1: Laboratory findings.
ANF: anti-nuclear factor; SSA: Sjögren’s-syndrome-related antigen A; SSB: Sjögren’s-syndrome-related antigen B; C3: complement component 3; C4: complement component 4; CRP: C-reactive protein; ds: double stranded; ESR: erythrocytes sedimentation rate; PCI: protein creatinine index.
The patient denied other lupus symptoms in addition to mild diffuse non-scarring alopecia, totalling 14 points in the European League Against Rheumatism (EULAR)/ American College of Radiology (ACR) 2019 classification criteria for SLE.4
Treatment was started with methylprednisolone 125 mg intravenously daily for 5 days, with complete symptom resolution. Afterward, the patient received daily doses of prednisone 60 mg, hydroxychloroquine 400 mg, and azathioprine 50 mg. There was a prompt resolution of proteinuria and haematuria, with the protein/creatinine ratio changing from 0.8 to 0.1. The patient was discharged asymptomatic, with oral diet and outpatient follow-up.
DISCUSSION
To the authors’ knowledge, this is the first case report of lupus enteritis as the initial presentation of SLE. This case of lupus enteritis was hard to diagnose because it occurred without a previous diagnosis of SLE. Its clinical presentation, consisting of abdominal pain, vomiting, diarrhoea, and weight loss, is non-specific and can be confused with several chronic gastrointestinal diseases, making it a challenge to diagnose even for specialised care teams in gastroenterology. In rheumatological practice, SLE may present with overlapping symptoms and laboratory findings with other immunological and non-immunological diseases, making it difficult to manage.5
Approximately 50% of patients with SLE present with gastrointestinal symptoms, particularly non-painful palatal ulcers.5,6 Lupus enteritis is a rare complication of SLE, diagnosed in 1% of patients with lupus with abdominal pain, and accounting for up to 65% of cases of acute abdomen in the presence of SLE.7 It has considerable mortality, particularly after delayed recognition.1,5 Its pathophysiology is still uncertain, but many authors believe that immunocomplex deposits are responsible for microvascular lesions, possibly causing intestinal ischaemia.8,9 Although the lesion is due to small vessel arteritis and venulitis in most cases, blood vessel inflammation is not found in all patients. Therefore, such a condition was named ‘lupus enteritis’ rather than gastrointestinal vasculitis.10
The clinical picture includes abdominal pain of sudden or insidious onset, nausea, vomiting, and chronic diarrhoea. Bowel necrosis and perforation may occur, usually associated with gastrointestinal bleeding, resulting in high mortality. Although it can affect any area of the digestive tract, the supply territory of the superior mesenteric artery is the most implicated.11 Furthermore, the predominant sites of intestinal involvement are the ileum (85%) and the jejunum (80%).8
Laboratory findings include haematological abnormalities, such as anaemia (52%), leukopenia and lymphopenia (40%), and thrombocytopenia (21%). The median C-reactive protein value was 2 mg/dL.1 About 88% of the patients had reduced complement levels, and proteinuria was present in almost half of them.1,3 In the present case, although the anti-double stranded DNA was negative, the diagnosis of lupus was made by positive anti-nuclear factor and anti-Sjögren’s-syndrome-related antigen A, consumed complement component 3 and complement component 4, thrombocytopenia, leukopenia with lymphopenia, sub-nephrotic proteinuria, and alopecia.
The abdominal X-ray is usually normal, but the ultrasound may demonstrate thickened intestinal walls and free fluid. The best image method is abdominal CT, which may reveal distended intestinal loops, focal or diffuse intestinal wall thickening, altered enhancement (halo sign), engorgement of mesenteric vessels (comb sign), and attenuation of mesenteric fat and ascites. The comb sign is present in approximately 70% of patients with lupus enteritis.12 Arteriography has limited value, since the disease affects small vessels.8 Endoscopy and colonoscopy serve to identify mucosal inflammation and perform biopsies, helping in the differential diagnosis.13
In cases with no evidence of intestinal perforation, the ischaemia is potentially reversible, favouring a conservative treatment. It includes high-dose corticosteroids (prednisone 1-2 mg/kg/day) or pulse therapy with methylprednisolone 1 g daily for 3 days. The authors’ patient improved quickly with a 5-day course of an intermediate dose of methylprednisolone (125 mg per day). Intestinal rest should be associated. Prokinetics can help by improving peristalsis and reducing intraluminal pressure.13,14 In patients resistant to corticosteroids, cyclophosphamide or mycophenolate can be used, but the latter presents a better safety profile.15 The authors treated their patient with azathioprine due to its accessibility and the rapid improvement that the patient had with corticosteroids, predicting a good response also to azathioprine. The mycophenolate was expensive, and cyclophosphamide, although potent, was left in the background due to its toxicity.16 In patients refractory to cyclophosphamide or other immunosuppressants, tacrolimus can be helpful, as described in case reports.17
The prognosis is generally good, and recurrence is infrequent.1,5 However, some factors may be associated with a higher recurrence rate, such as colonic or urinary tract involvement, intestinal wall thickness >9 mm, and low cumulative dose of immunosuppressants.2,14
It is important to note that one of the main limitations observed in the authors’ case report would be the short follow-up for this patient, which was about 4 months.
CONCLUSION
In conclusion, the authors reported a case of lupus enteritis as the first presentation of SLE, with a difficult diagnosis but a remarkable response to immunosuppressive treatment. They emphasised the clinical manifestations of lupus enteritis, its pathophysiology, and its importance to differential diagnosis in cases with abdominal pain and chronic diarrhoea. The authors highlighted the complex diagnostic tools and the importance of proper management.







