Abstract
The end of the 20th and the beginning of the 21st centuries witnessed a change in human nutritional status, from malnutrition to an obesity pandemic. During a similar time period, many Western-type, non-communicable diseases increased globally in previously low-incidence areas. One group, inflammatory bowel diseases (IBD), is particularly noteworthy because its treatment does not eliminate the disease. Its current pan-global rise in incidence and prevalence seems to follow regions with more recent increases in prevalence of obesity. Obesity, which has now been redefined as a chronic disease, seems to share some pathogenic features with IBD. These include the promotion of a pro-inflammatory state via fat-derived hormones and oxidative stress induced by insulin resistance. In addition, the intestinal microbiome in obesity and IBD may also contribute to mutual disease development. Finally, the merger of these two diseases impacts the clinical course of IBD. This review explores these relationships.
INTRODUCTION
The end of the 20th and the beginning of the 21st centuries witnessed a remarkable change in human nutritional status. Prior to this period, a large portion of the world’s population experienced malnutrition; however, the subsequent four decades witnessed global increases in body weight. While poor nutrition and being underweight are associated with poor health, being overweight and obesity are also associated with multiple health issues. The National Institute of Health (NIH) recognised obesity as a chronic disease rather than a behavioural disorder in 1998.1 Specific biochemical, physiological, and microbiomial abnormalities have been described. Furthermore, the pathogenic importance of the intestinal microbiome (bacteria, archaea, fungi, and viruses) has emerged as an important factor in many diseases.2,3 It is then plausible that obesity can merge and promote diseases linked with disturbances in the microbiome.
Inflammatory bowel diseases (IBD), consisting of Crohn’s disease (CD) and ulcerative colitis (UC), are immunologically mediated conditions in conjunction with disruptions of the microbiome.4 More recently, IBD has been reported to be associated with comorbidities found in obesity.5 In addition, the geographic expansion of IBD, to some extent, follows the obesity pandemic. This review will outline reported relations between these two diseases.
OBESITY: THE PANDEMIC
Obesity is generally defined by the World Health Organization (WHO) with a formula relating body weight in kg to height in m2.6 By this definition, overweight is defined as 25–29 kg/m2; Grade I obesity as 30–<35 kg/m2 (in Asia it is considered ≥27 kg/m2); Grade II obesity as 35–<40 kg/m2; and Grade III obesity (morbid obesity) as ≥40 kg/m2. Progressive increase in weight adds additional risks, which are also modified by the distribution of body fat. A central location of abdominal fat (android distribution, which is more common in males) confers higher risk for complications than a more diffuse distribution (pear-shaped obesity and gynoid fat distribution, which is more common in females).7
Obesity promotes the metabolic syndrome consisting of diabetes, hypertension, sleep apnoea, dyslipidaemia, and chronic fatty liver. In turn, these conditions lead to complications of the cardiovascular system, renal disease, advanced liver dysfunction, as well as hepatocellular carcinoma.8 In addition, obesity is associated with a number of gastrointestinal disorders and cancers.9
The pandemic of obesity began in Western countries, primarily in the USA, towards the end of the 20th century10 and is predicted to continue to spread globally well into the first third of the 21st century.11
The causes for obesity are linked to excess caloric intake with reduced energy utilisation. However, dietary factors do not completely explain the difficulties with weight reduction and resistance. An increasing body of information has been compiled on the role of environmental polluting compounds in industrialised societies, ‘obesogens’, which alter energy homeostasis.12 Furthermore, approximately 300 polymorphisms have been discovered by genome-wide association studies to be operational in obesity.13 These are thought to interact with environmental variables (e.g., diet, which is the easiest to change, lack of exercise, or other factors).13
Once obesity is established, a pro-inflammatory reaction is induced. This state is achieved by interactions between fat cell-derived and other hormones and the promotion of insulin resistance. Among several hormones, subcutaneous-derived leptin, which also controls appetite, is increased in obesity and promotes the release of inflammatory cytokines. Adiponectin, from visceral fat cells, counteracts leptin but control is diminished as this hormone secretion is reduced.14 These hormones induce insulin resistance. Additional hormones, such as resistin derived from the r eticulo-endothelial system, also contribute.15 In the pro-inflammatory response, TNFα inhibits tyrosine kinase at insulin receptors, conferring resistance and leading to enhanced oxidative stress.16
Additional nutrient-derived energy is provided to the host by the intestinal microflora.17 Alterations in distribution, diversity, and richness from normal, or dysbiosis, is a term describing bacterial changes that can lead to or are a result of host disease.2,18 The microbiome is responsive to external effects related to human culture, diet, antibiotics, and the changing local environment, much of which is related to industrialisation.19
As outlined above, pathogenic factors that underpin obesity also have profound effects on numerous diseases that have been labelled as ‘Western’ civilisation diseases.20 Among these, of note is the global spread of IBD into areas previously devoid of or reporting only very low incidence rates. Figure 1 outlines possible pathogenic mechanisms that might mutually interact to produce diseases.
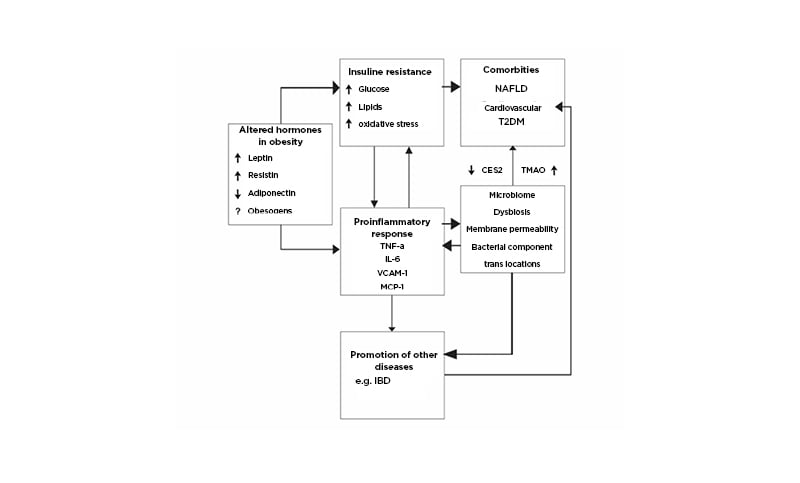
Figure 1: Pathogenic relationships of obesity with comorbidities and the potential for complicity in other illnesses such as inflammatory bowel diseases.
Obesogens are compounds polluting the environment and altering metabolic homeostasis (e.g., polycyclic aromatic hydrocarbons, polybrominated diphenyl ethers, and others).12 Pro-inflammatory cytokines, TNFα, IL-6, VCAM-1, MCP-1, CES243 promote NAFLD, TMAO55 through diet, and bacterial metabolism promotes atherosclerosis and increases risk for cardiovascular disease.
CES2: carboxylesterase-2; IBD: inflammatory bowel diseases; MCP-1: monocyte chemoattractant protein-1; NAFLD: non-alcoholic fatty liver disease; T2DM: Type 2 diabetes mellitus; TMAO: trimethylamine N-oxide; VACM-1: vascular cell adhesion molecule-1.
Adapted from Szilagyi A.22
INFLAMMATORY BOWEL DISEASES AND EXPANDING INCIDENCE
UC and CD are two related, yet different, immune-mediated diseases of the colon and intestines. Both diseases have been associated with progressive Western industrialisation.21 There is no cure for IBDs and, although they are associated with relatively low mortality, IBDs are generally lifelong conditions with relapsing clinical flares.
UC, limited to the colon, was recognised earlier, while CD, potentially involving the entire gastrointestinal tract, was described in detail in the first half of the 20th century. The causes of IBD are complex. While there are differences between UC and CD the current paradigm is the interaction of the role of host genetic factors, which lead to inappropriate immune responses in conjunction with a disturbed balance in host microbial flora. A variety of environmental factors are considered related to IBD development.23
These IBDs were also initially described in Western societies. Incidence rose somewhat in the second half of the 20th century. Subsequently, from the early 1950s to the end of the 20th century, IBD expanded in Western cultures and began to stabilise, except in paediatric populations.24
The incidence of UC and CD began to increase in regions of the world previously free of IBD by the end of the 20th century and has risen progressively since. There are some epidemiological differences in IBD in different parts of the world (such as Asia, South America, and Africa).25,26 Based on the accumulated tracking of IBD from various regions, Kaplan and Windsor21 hypothesised a four-stage evolutionary development of IBD: an early stage of emergence; accelerated incidence followed by a balance between incidence and prevalence; compounding incidence; and, finally, when a balance between incidence and prevalence is reached, prevalence equilibrium.21
RELATIONS BETWEEN OBESITY AND INFLAMMATORY BOWEL DISEASES
Epidemiology
Although both obesity and IBD emerged from Western industrialised societies, geographic expansion of IBD and obesity diverge. IBDs, like several other Western civilisation diseases,19 were observed to follow diminishing latitudes toward the equator.27,28 This was initially ascribed to increasing exposure to sunshine and, hence, vitamin D skin synthesis. In turn, new research showed that vitamin D has pleiotropic anti-inflammatory and anti-neoplastic functions.29 As many of these diseases were associated with low serum vitamin D, the lack of sunshine exposure was hypothesised to be the cause for higher incidence rates at higher latitudes. Although obesity was also observed to be associated with low vitamin D,30 its trajectory included a geopolitical West to East direction.11
Perhaps co-incidentally, the incidence of some of Western civilisation diseases including IBD also inversely correlated with increasing population proportions who were unable to digest lactose.31 Lactose digestion in adulthood is a dominant genetic trait that occurred in human evolution some 7–10×103 years ago.32 The genetic trait divides humanity mainly into two phenotypes of lactase-persistent and lactase-non-persistent people (LNP).
On a global scale, obesity is modestly and inversely correlated with LNP status and more weakly but positively with latitude. This relationship of obesity with IBD may interfere with previously observed north–south (south–north) relations with IBD,33 a topic that was of interest in the late 20th century.
The second epidemiological variation is the relationship of economic growth with increasing rates of IBD. Initially, both obesity and IBD were associated with wealthy countries. Obesity was found more in rural communities but, with time, obesity became associated with poorer economic areas in crowded urban locations.10 Similarly IBD is associated with more urban crowding but generally higher socioeconomic standards.23,26
This association with national economic growth and industrialisation, as defined by the Gross National Product (GDP), has also been reported.26,34 Globally, GDP correlates positively with latitude and negatively with LNP distributions, similar to IBD. However, correlation of obesity diverges from IBD and GDP.34 Regional relationships, as in China, retain the close associations of IBD with GDP but are more evident in a south- to north-west direction from coastal areas.26 Overall, in Asia all these relationships are negligible.
These findings could suggest that obesity is more dependent on adoption of a Western-type diet, which may precede independently from national economic growth.10,35 It also suggests that IBD is associated with other features of acquisition of national wealth besides diet.
Mutual Pathogenic Influences
As described above, obesity promotes a pro-inflammatory milieu through insulin resistance and adipokine-mediated imbalance. Hypothetically, this state could favour any disease where pro-inflammatory cytokines contribute to pathogenesis.
In addition to physiological parameters, as pointed out, intestinal microbial changes likely contribute to obesity, IBD, and other diseases. The microbiome is intimately affected by diet. High fats such as saturated, monosaturated, polyunsaturated, and linoleic acids play roles in modulating the immune system. Western-type diets lead to obesogenic and pro-inflammatory patterns of the microbiome.34 Although there is no clear evidence that a low-fat diet leads to more weight loss than a high-fat diet,36 similar high-fat diets play a probable role in inducing and possibly causing relapses in IBD.37,38 High carbohydrates, especially refined sugars, also contribute to a pro-inflammatory state, through the contribution to insulin resistance. However, fibres from vegetables and, to an extent, fruits are considered beneficial in IBD.23 In obesity, fibres are helpful but fructose may be harmful. Proteins, particularly in dairy foods, may be protective against cardiovascular events, helping to maintain better weight control and reducing the risk of IBD before overt disease development.39 Some similar effects of diet are shown in Table 1.
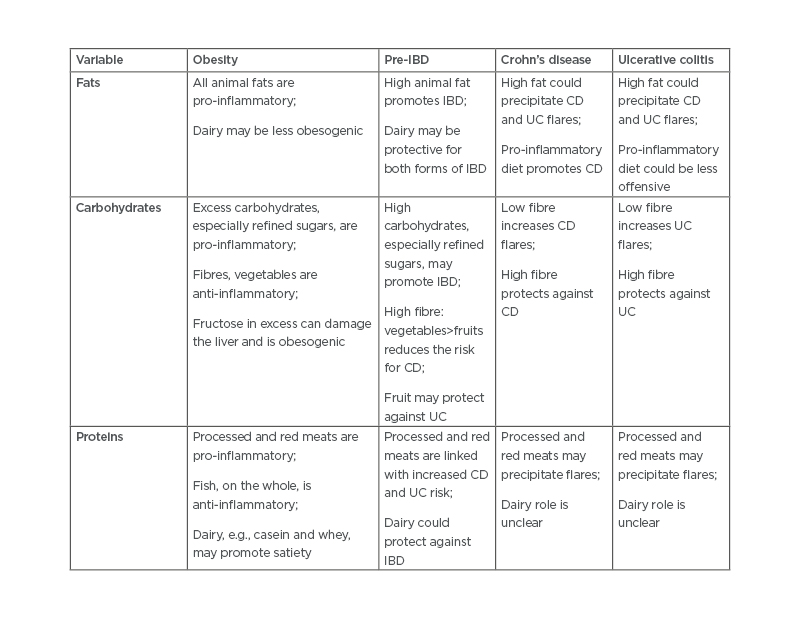
Table 1: Summary of current hypotheses related to dietary promotion and prevention of obesity and both forms of inflammatory bowel diseases.23,34,36-39
CD: Crohn’s disease; IBD: inflammatory bowel disease; UC: ulcerative colitis.
Consumption of pro-inflammatory nutrients could lead to alterations in gut barrier tight junctions, with bacterial access to the host immune system.
A microbiome pattern that is altered is referred to as dysbiosis.2,18 Although microbiome findings vary in different studies, recently described bacterial milieu in IBD shares a number of similarities with obesity as described in Table 2.22 In IBD, strictly anaerobic microflora are replaced to an extent by facultative bacteria. The similar sharing of bacterial characteristics could, hypothetically, lead to mutual predisposition between diseases. Indeed, the long-term follow-up of patients, especially with CD, show increasing weight gain over time.40
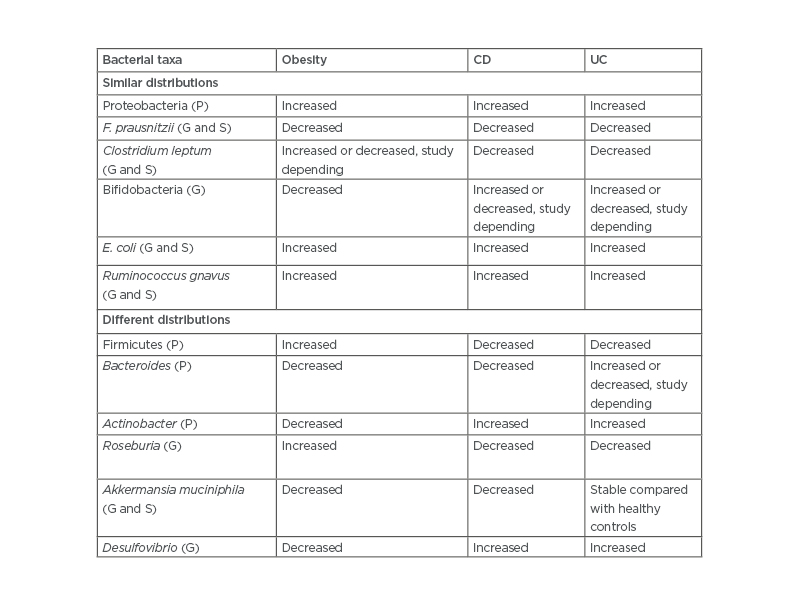
Table 2: Comparison of bacterial abundance in the microbiome of patients who are obese, with Crohn’s disease, or with ulcerative colitis.
CD: Crohn’s disease; E. coli: Escherichia coli; F. prausnitzii: faecalibacterium prausnitzii; G: genus; P: phyla; S: species; UC: ulcerative colitis.
Szilagyi A22
Shared Comorbidities
The apparent shared pathogenic similarities between obesity and IBD also lead to increasing shared comorbidities. Among these, non-alcoholic fatty liver disease (NAFLD) is the best described. This condition, defined as >5% of liver fat, is largely attributed to insulin resistance, which leads to altered lipid metabolism and transport out of the liver. The role of TNFα interfering with insulin receptors is thought to lead to resistance and hence connect pro-inflammatory-dependent disease on predisposition to NAFLD.16 This mechanism is thought to be similar in individuals who are both overweight and normal- or underweight.41 A recent study showed that reduced carboxylesterase 2, which affects diglyceride and monoglyceride metabolism in the intestine and liver, could contribute to NAFLD in patients with obesity and CD alike.42
World rates of NAFLD are reported to be 9–43%. In the USA, 27% of the population is reported to have NAFLD. The risk of NAFLD is reported to be 3.5 times higher in people who are obese.43 In IBD, a systemic review found that 39.5% of patients with CD and 23.0% of patients with UC had NAFLD compared with 20.0% (range: 6.0–33.0%) of controls.44 In a prospective study of 321 patients with CD, followed for 3 years, one-third developed NAFLD, although <1.0% were considered obese. Of these, 2.2% developed more advanced liver disease over time, such as non-alcoholic steatohepatitis, inflammation, ballooning degeneration, or degrees of fibrosis.45 Another study of patients with IBD reported a prevalence of 32.8%, of which 12.2% were advanced; in this case, 30.4% of patients were overweight and 13.8% were obese (BMI: ≥30kg/m2).46 The impact of the microbiome on altered intestinal barrier and bacterial translocations are also thought to play an important role in NAFLD development.47
Another shared comorbidity between obesity and IBD is cardiovascular complications. However, the risk factors in IBD differ somewhat from those observed in obesity. The types of vascular problems are summarised in a recent review and include venous and arterial thromboembolism; heart failure; arrhythmias; valvulopathies, with or without endocarditis; and Takayasu arteritis.48 The occurrence of ischaemic stroke or myocardial infarction is somewhat controversial. An earlier meta-analysis did find increased episodes of myocardial infarction in IBD,49 while another reported study reported inverse association of cardiovascular disease (CVD) with IBD.50
However, a more recent meta-analysis did find an increased, albeit modest, risk for cardiovascular events in both CD and UC, interestingly more frequently in women aged <50 years. These events were unaffected by smoking and controlling for obesity.51
In obesity, traditional risk factors related to adipocytes and insulin resistance promote atherosclerosis52 and cardiovascular complications. It is of note that in Class I obesity (but less so in Classes II and III), prognosis for cardiac event outcomes is better than in patients who are of normal weight or lean and with CVD. This has been called the ‘obesity or lean paradox’.53
The role of microbial contributions to CVD comorbidities in obesity have been evaluated by examining plasma metabolites in a cohort of patients who are obese and compared with controls who are not obese. In this study, 48 metabolic microbial pathways were linked with CVD risks.54 In addition, dietary intake of choline and carnitine (found in animal products) are metabolised by bacteria to produce trimethylamine, which is oxidised in the liver to become trimethylamine N-oxide. This compound increases platelet adhesiveness and promotes atherosclerosis.55 A similar effect could contribute to CVD in IBD, depending on diet.
The recent increasing association of Type 2 diabetes mellitus (T2DM) with IBD adds another measure of overlap between obesity and IBD. In obesity, insulin resistance leads to T2DM via the promotion of a pro-inflammatory state. It is suggested that the inflammatory milieu of IBD contributes to insulin resistance as well. One study reported a modest 26% increase in T2DM in UC after an 11-year follow-up compared with healthy controls.56 A more recent report from Korea, however, found that T2DM was more frequent in CD (hazard ratio: 1.677; 95% confidence interval: 1.408–1.997), but not in UC. The occurrence was also more common in younger patients.57However, a recent Danish study, based on more than 6 million persons followed for 37 years, found increased risk (by 54–57%) for T2DM in both CD and UC. Notably the risk increased after 2003 compared to the time period from 1977.58 This time difference is of note because the obesity pandemic is hypothesised to have begun around the 1980s.10
CLINICAL IMPACT OF OBESITY ON INFLAMMATORY BOWEL DISEASE
If these two diseases collide in time and geography, what effects of obesity can be seen on the clinical course of IBD? The influence of obesity on IBD location is not well defined. A population-based study of 488 patients found a trend in favour of colonic involvement,59 while another study found increased perianal disease and more hospitalisations in CD,60 the previous study58 did not substantiate this.
Obesity was reported to increase risk of surgery in UC but decreased in CD,61 and was reported to be more frequently associated in women with UC.62 In that study, metabolic syndrome and C-reactive protein were increased but were not related to BMI.63 However, in another report, the presence of metabolic syndrome was found to increase the risk of hospitalisation two-fold.63 This contrasted with another study that found that obesity was associated with a more mild course of CD.64 A meta-analysis of 7 studies (5 UC and 2 CD), reported that patients who were obese were less likely to undergo IBD-related surgery, receive hormonal therapy, or require hospitalisation than patients who were not obese.65 The presence of obesity and traditional associated comorbidities may increase perioperative complications, although not all reports agree; these are reviewed by Johnson and Loftus Jr.66
There is also a debate over whether patients with IBD who are obese respond as effectively to biologic therapy as patients who are not obese. In the case of TNFα inhibitors, reduced clinical outcome was reported in patients with IBD who were obese.67 However, another study failed to support therapeutic failure, and a meta-analysis of therapeutic response to biologics in a number of autoimmune diseases found a 60% higher failure rate but not in IBD.68 In addition, a report suggested that the α4β7 integrin inhibitor vedolizumab trough levels were lower in patients with IBD who were obese.69
EVIDENCE THAT OBESITY PROMOTES INFLAMMATORY BOWEL DISEASE
Despite the theoretical similarities in pathogenesis between obesity and IBD, the literature is conflicting on a clear evolution between the two diseases. There are emerging hypotheses suggesting an interchange between these two diseases, despite the presence of malnutrition in IBD in the past.
Mendall et al.70 initially suggested that obesity may promote CD. In a cohort of 524 patients, obesity at diagnosis was linked more with CD than UC or healthy controls.70 More recent studies showed that obesity at the age of 18 years increased the risk for CD but not UC in the Nurse’s Health Study II.71 These reports were similar to previous findings.70 In two other Danish studies, one found that increasing BMI in children between the ages of 7 and 13 years increased the risk for CD until age 30 years, but there was an inverse relationship with UC.73 In the second study of young military conscripts, there was a ‘U’-shaped risk profile for CD up to the age of 60 years. However, only a low BMI (<18.5 kg/m2) was statistically significantly associated with CD. This study also showed an inverse association with UC.73
As stated above, the reverse observations have also been reported, that patients with CD enrolled in clinical trials gain weight with time over follow-up.39
SUMMARY AND CONCLUSIONS
In this review, the obesity pandemic has been examined together with the coincidental extension of both forms of IBD, UC and CD, into areas of previous low incidence. Both diseases, with differences in detail, are linked with a pro-inflammatory state. A large part is related to the functions and alterations in the intestinal microbiome. In addition, adipocytes in obesity contribute to the pro-inflammatory pattern. Although genetic predispositions differ, the internal milieu in each disease leads to similar development of comorbidities such as NAFLD, some cardiovascular complications, and T2DM. As such, there are suggestions that some features of obesity and IBD are interchangeable. That is, the pathogenic changes in IBD can lead to weight gain and, under some circumstances, obesity may promote IBD. These interactions favour CD more than UC.
The consequences of these interactions could lead to variations in clinical outcomes in IBD. It is not yet clear whether a ‘paradoxical’ effect in IBD occurs as it has been described in CVD. On an epidemiological level, it is hypothesised that the geographic distribution of obesity impacts on patterns of IBD, which is best seen in Europe. This impact may partly have led to the disruption of previously observed north–south disease distributions there. Secondly, although obesity and IBD both emerged in Western societies, often in wealthier, industrialised regions, the two diseases diverge in their expansion somewhat. In areas where obesity is evident and not accompanied by increased economic wealth, IBD rates are proportionately lower. However, IBD may be closely following areas affected by increased obesity. Thus, there may be a subtle interaction evident that promotes both conditions. While ‘Western’ diets may precede economic growth, chemical pollutants may affect these interactions in ways that are not yet clear. Seen in this light, obesity may be a marker for impending regional development of IBD (and some other civilisation diseases).








