Abstract
Selective angioembolisation is a common treatment modality for lower gastrointestinal bleeding. Sepsis is an uncommon complication of the procedure, and infective endocarditis is even rarer yet. Presented here is a case of a male in his 50s who received selective angioembolisation to a branch of the right colic artery to control lower gastrointestinal bleeding. He subsequently developed sepsis and acute cardiac failure due to Enterococcus faecalis bacteraemia and infective endocarditis, requiring a valve replacement surgery and an extended course of antibiotics.
Key Points
1. Selective angioembolisation is a common and safe treatment modality for lower gastrointestinal bleeding. Sepsis is an uncommon complication of the procedure, and infective endocarditis is even rarer yet.
2. This is a case report of native aortic valve infective endocarditis following selective angioembolisation for lower gastrointestinal bleeding.
3. Bacteraemia and infective endocarditis are rare but important differential diagnoses to consider in a patient presenting with sepsis following gastrointestinal intervention.
INTRODUCTION
Selective angioembolisation is a safe and minimally invasive technique for the treatment of acute lower gastrointestinal (GI) bleeding. It is a valuable alternative treatment modality for patients who are poor surgical candidates, or refractory to transfusion resuscitation or endoscopic treatment. Technical success of this procedure has been reported to be 85–100%. Well recognised complications include rebleeding (0–20%), ischaemic complications to the bowel (0–7%), acute kidney injury, and access-site complications.1 The authors report a rare case of native aortic valve infective endocarditis following selective angioembolisation for lower GI bleeding.
PATIENT INFORMATION
A male aged in his 50s presented to the authors’ emergency department following a syncopal episode associated with rectal bleeding and vomiting without haematemesis. He denied any abdominal pain, recent change in bowel habit, weight loss, or constitutional symptoms. He was otherwise previously well and did not take any regular medications. He had a colonoscopy 2 years prior, which showed widespread diverticular disease throughout the colon, and had previously had a laparoscopic appendicectomy for appendicitis.
Clinical Findings
The patient was haemodynamically stable, and his abdominal examination was unremarkable. Digital rectal examination was painless, with blood-stained mucus on the glove. Laboratory testing revealed he had anaemia with a haemoglobin of 94 g/L. His renal function and liver function were normal.
Diagnostic Assessment
He was admitted to the authors’ surgical unit for observation and was placed on a clear fluid diet. The following day, he had a further episode of large frank rectal bleeding, with a significant drop in his haemoglobin to 74 g/L. At this time, he reported light-headedness and abdominal discomfort. An urgent CT mesenteric angiogram showed an active blush in the ascending colon, which was presumed diverticular in origin. There were no
signs of colitis.
Therapeutic Intervention
The patient was resuscitated with three units of packed red bloods cells and his haemoglobin incremented to 92 g/L. He underwent urgent selective angioembolisation with digital subtraction angiography, performed by the interventional radiology department (Figure 1). A branch of the right colic artery was embolised using two Tornado® embolisation coils size 3/2 (Cook Medical, Bloomington, Indiana, USA) and this successfully controlled the bleeding. He had two episodes of self-limiting rebleeding 24 hours later, and was treated with further packed red blood cell transfusions. He was then discharged with outpatient follow-up.
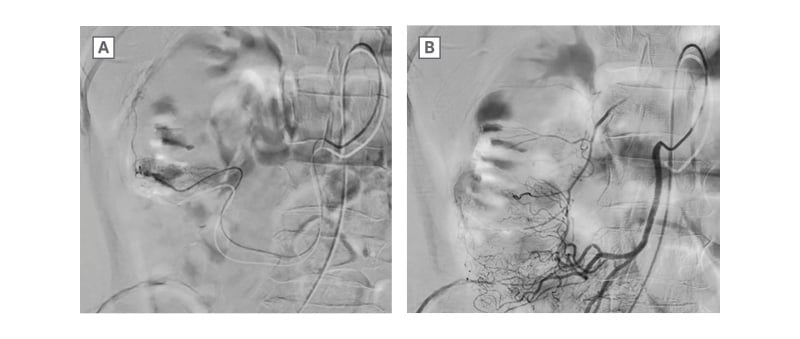
Figure 1: A) Digital subtraction angiography showing contrast extravasation ‘blush’ from a branch of right colic artery. B) Post embolisation, coils shown in situ and resolution of the arterial blush.
Eighteen days later the patient re-presented with fevers and dyspnoea. Blood cultures returned a result of Gram-positive cocci within 24 hours. On examination, there were new pansystolic and diastolic murmurs, and peripheral oedema. Clinically, the patient was in decompensated heart failure. There were no peripheral stigmata of infective endocarditis. A chest X-ray was performed, which showed features of pulmonary oedema. He had no other focus of infection noted on history, examination, and investigations. Treatment with intravenous benzylpenicillin 2.4 g 6-hourly was commenced until identification of Enterococcus faecalis on blood cultures. At this point, the antibiotic therapy was changed to intravenous ampicillin 2.0 g 6-hourly and ceftriaxone 2.0 g 12-hourly. Transoesophageal echocardiogram revealed flail aortic valve leaflet with torrential aortic valve regurgitation and findings suspicious for an aorto-atrial fistula. No obvious vegetation was identified and coronary angiogram was normal.
The patient underwent an emergency bioprosthetic aortic valve replacement with a 25 mm INSPIRIS RESILIA bioprosthesis (Edwards Lifesciences, Irvine, California, USA). Intraoperative findings showed an infected aortic valve, with the left coronary cusp destroyed by vegetations; the non-coronary cusp had a vegetation with perforation; and the right coronary cusp was normal. There was no aorto-atrial fistula.
He was admitted to the ICU post-operatively and was transferred to the surgical ward on post-operative Day 2. Tissue culture of the aortic valve vegetations confirmed growth of E. faecalis that was sensitive to ampicillin, gentamicin, and vancomycin. Antibiotic therapy was changed to intravenous ampicillin 2.0 g 6-hourly and gentamicin 60.0 mg 12-hourly, as per susceptibility testing. A peripherally inserted central catheter line was inserted to facilitate long-term intravenous antibiotic therapy.
Follow-up and Outcomes
The patient was discharged home on post-operative Day 14. Antibiotic therapy in the community setting comprised intravenous gentamicin 60.0 mg twice daily and benzylpenicillin infusion of 14.4 g over 24 hours. Metoprolol and aspirin were commenced as long-term therapy. Five days after discharge, gentamicin was changed to ceftriaxone 2.0 g 12-hourly due to a possible new, mild vestibulocochlear toxicity noted on examination during outpatient follow-up. In total, the patient completed 6 weeks of intravenous antibiotic therapy from the date of the operation and
was discharged from the clinic.
DISCUSSION
To the best of the authors’ knowledge, the present case is the first reported case of infective endocarditis as a complication of angioembolisation to control lower GI bleeding. The presumed origin of the E. faecalis bacteraemia in this case was the GI tract, given the temporal association between the angioembolisation and infection, and the absence of other risk factors for infective endocarditis. Enterococci are Gram-positive facultative anaerobic bacteria that are normal commensal bacteria in the human GI tract.2 In the healthy state, the intestinal mucosal barrier provides physical, biochemical, and immunological defence against bacterial translocation. Insults causing tissue injury, such as ischaemia and surgical manipulation of the intestine, can cause compromise to this protective barrier.3 Bacterial translocation of enterococci species from the GI tract to the bloodstream and lymphatics can result in bacteraemia, sepsis, and seeding of distant organs. Patients with E. faecalis bacteraemia are at high risk of infective endocarditis. In a study by Dahl et al.,4 26% of 344 patients with E. faecalis bacteraemia were found to have infective endocarditis on echocardiography. Indeed, infective endocarditis may lead to potentially fatal complications including systemic embolisation, cardiac failure, mycotic aneurysm, and neurological complications.5
The authors propose that in the case presented in this article, local ischaemia from angioembolisation resulted in disruption of the intestinal mucosal barrier, thus allowing translocation of enteric bacteria into the systemic circulation.6-8 The extent of the vascular bed embolised should be considered, particularly as even subclinical ischaemia-reperfusion injuries can result in bacterial translocation.9 It would be logical to hypothesise that the larger the vascular bed embolised, the larger the area of potential ischaemic bowel injury. The risk may be reduced with superselective angioembolisation, where the arterial recta supplying the site of haemorrhage is catheterised and embolisation performed only if the bleeding source is identified.10
In the presented case, prophylactic antibiotics were not administered during selective angioembolisation for lower GI bleeding. The patient had no identifiable risk factors for the development of post-procedure infective endocarditis, as outlined by the Society of Interventional Radiology (SIR). Current guidelines indicate that antibiotic prophylaxis for embolisation of GI bleeding is not necessary, except in cases of haemobilia.11 Additionally, the American Heart Association (AHA) guidelines indicate that antibiotic prophylaxis solely to prevent infective endocarditis is not recommended for patients undergoing GI procedures.12
In the case presented, the patient had a history of underlying diverticular disease, diagnosed on a colonoscopy performed 2years prior. Escolà-Vergé et al.13 have demonstrated that existing colonic disease is very common amongst patients with E. faecalis endocarditis, even if the portal of entry is presumed to be known. In their study, colorectal disease was found on colonoscopy following diagnosis of E. faecalis endocarditis in 60% of cases.13 Therefore, it is possible the history of underlying diverticular disease and certainly the acute GI bleeding were important predisposing factors in the case presented in this article. Perhaps there may be a role for antibiotic prophylaxis for lower intestinal GI angioembolisation in cases with high-risk features, such as pre-existing colonic pathology or active bleeding.
There is still much to be explored regarding the role of the gut microbiome and dysbiosis in states of health and disease. Translocation of E. faecalis has been found to coincide with a threshold of enterococcal colonisation in the gut lumen in mouse models.14 E. faecalis overgrowth may also stimulate reactive oxygen species production and colonic cell genomic instability. Reactive oxygen species are known to play a role in the pathogenesis of intestinal ischaemic injury, thus increasing the risk of bacterial translocation.15,16 As such, it can be hypothesised that gut dysbiosis may predispose some individuals to the risk of bacterial translocation and the associated complications.
CONCLUSION
This case illustrates an unusual and life-threatening complication of selective angioembolisation to control lower GI bleeding. Clinical features of bacteraemia and infective endocarditis may initially be vague; however, they are important differential diagnoses to consider in a patient presenting with sepsis following GI intervention. Compromise of the intestinal mucosal barrier may predispose to the translocation of enteric bacteria into the systemic circulation, which could lead to potential distant colonisation.
Informed Consent
The patient provided informed consent for de-identified details of this case to be published.







