Abstract
Objective: Recent studies have shown a potential link between the gut microbiome and colorectal cancer (CRC). A wide array of research into this topic was performed over the past decade, illustrating a keen interest in the potential causal relationship between the gut microbiome and CRC. However, the cancer research community is lacking a concise review of this kind, which aims to explore the evidence linking the human gut microbiome to the risk of developing CRC.
Design: This narrative review was carried out by two independent reviewers who assessed the database outcomes from Medline and EMBASE during May 2020. A meta-analysis was undertaken to study the link between Helicobacter pylori and CRC. The meta-analysis was processed through Stata (StataCorp LLC, Lakeway Drive, College Station, Texas, USA).
Results: Thirty one papers were included in this narrative review, of which 12 were included in the meta-analysis. From these papers, Fusobacterium and Bacteroides were reported most frequently as enriched in those with CRC versus the control populations. The meta-analysis showed an odds ratio of 1.49 (95% CI: 1.19–1.86), including a total of 20,001 events. This meta-analysis concluded that H. pylori infection significantly increases the risk of CRC, albeit with evidence of publication bias.
Conclusion: Bacteria have been found to increase the risk of CRC; however, a definitive causal relationship cannot be concluded or excluded using case-control studies. To fully understand the potential link of the bacteria listed, alterations in research design and execution are required. The assessment found a need for a large-scale cohort study conducted over a significant period of time to thoroughly evaluate the potential relationship between gut microbiome and CRC risk.
Key Points
1. In the UK, 46 people per day die from colorectal cancer (CRC), with it being the third most common cancer worldwide. Increasingly, CRC has been linked to the gut microbiome, with certain genera/species linked to an increased risk of developing the disease.
2. This narrative review and meta-analysis investigated the link between CRC and the gut microbiome, with Helicobacter pylori being the subject of the meta-analysis.
3. This paper found a strong association between Fusobacterium and Bacteroides, amongst other genera, and CRC.
This meta-analysis also highlights a statistically significant association between Helicobacter pylori and CRC.
This may have great implications for future screening for CRC risk and provide a base for future research
into the impact of the gut microbiome on CRC risk and potential for probiotics for risk reduction.
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer worldwide, accounting for 10% of all cancer deaths in the UK during the period of 2015–2017.1,2 The global burden of colorectal cancer is expected to substantially increase in the next two decades, as a result of the widening adoption of a Western lifestyle.3 Cancer Research UK estimates that 54% of CRC cases are preventable, with many studies looking into the impact that lifestyle and other preventable factors such as red meat consumption, fibre intake, and obesity, can have on CRC risk.4-7
Recently, the risk of developing CRC has been closely linked to the composition of the gut microbiome, with many papers stating evidence for and against certain commensal species normally found in the human gastrointestinal (GI) tract. It has been suggested that an understanding of this gut flora composition offers potential in terms of the identification of biomarkers and associated risk factors for early CRC.8 This may have an important impact on the future personalised management of patients, potentially improving prognoses.
The aim of this review was to determine what genera/species of bacteria in the human gut microbiome are significantly linked to increased or decreased risk of CRCs, through the evaluation of papers that have been published on this subject. The review presented here summarises the bacterial taxa associated with altered risk of CRC, in line with this paper’s research question and the PRISMA statement.9
METHODS
Data Sources and Search Strategy
This review was reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The methods used were agreed by the authors in advance:
- Develop research question
- Identification of papers
- Screening
- Critical appraisal
- Data extraction
- Narrative synthesis
- Meta-analysis
Studies that reported the association between CRC and the gut microbiome were gathered from Medline and EMBASE, with the searches adapted to utilise relevant subheadings on each database. The following variations of keywords and MeSH terms were used: colorectal, cancer, neoplasm, tumour, malignancy, carcinoma, bacteria, microbiome, gastrointestinal, colonic, faecal, gut, dysbiosis.
Selection Criteria
In accordance with the population, intervention, controls and outcomes (PICO) proforma, the criteria used for the search and selection of papers for inclusion and exclusion in this review can be found in Supplement 1. Both reviewers agreed to base the exclusion criteria around factors that may alter the natural composition of the human gut microbiome, such as underlying conditions or medication exposure, which may inadvertently impact the composition of species. For example, a twin study by Willing et al.10 in 2010 demonstrated a significant difference in the gut microbiome of patients with inflammatory bowel disease when compared with their healthy twin, suggesting a potential for underlying conditions to alter the host microbiome.
The literature search, selection, and review were performed by two independent reviewers. Papers were removed from the selection if both reviewers agreed to exclude them at the various screening levels (title and abstract and full article eligibility), or if duplicates were found. In instances of disagreement, a resolution was reached via discussion between the two study team members. The overarching objective of the reviewers at this stage was to focus on the two absolutes of CRC risk and the gut microbiome, minimising the effect of potential microbiome-altering effects outside of these parameters (e.g. chemotherapy use, antibiotics, etc.).
Data Extraction
Data extraction was carried out independently by the two reviewers following the full article assessment and data were documented on an original electronic data extraction table. Extracted data included: author name, publication year, population location, population size, detection method, taxa enriched in CRC, and taxa enriched in control. The same data were extracted for the meta-analysis, with the number of Helicobacter pylori positive and negative patients documented for CRC, and healthy groups replacing taxa documentation.
Critical Appraisal
Two reviewers performed a critical appraisal of the selected articles. The relevant Critical Appraisal Skills Programme (CASP) checklist was used for cohort and case-control studies. The appropriate Newcastle-Ottawa Scale (NOS) was used for cross-sectional studies.
Main Outcomes Measured
Significantly raised (p<0.05) levels of bacterial taxa in patients with and without CRC were recorded in the data extraction table. The meta-analysis was specific to H. pylori presence in patients with and without CRC.
Meta-Analysis
During the screening and eligibility phases of this review, it became apparent that several of the articles studied the association between H. pylori and CRC. To further infer significance and verify this link, a decision was made to perform a meta-analysis on the eligible papers generated from the documented search strategy. To perform the meta-analysis, StataSE 16.1 for Mac (StataCorp LLC, Lakeway Drive, College Station, Texas, USA) was used.
Significance of association was measured using odds ratio (OR) and 95% CI. A random-effects model was chosen to analyse the data due to the differences amongst studies, namely in study design. Funnel-plot and contour-enhanced funnel-plots were generated to test for publication bias, alongside the use of Egger’s test and the trim-and-fill method.
RESULTS
A total of 1,058 abstracts were screened from Medline and EMBASE. The final count of articles included for this review and meta-analysis were 31 and 12, respectively. The process and numbers are documented in Figure 1.
Review
Study characteristics
A total of 30 out of the 31 articles included in this review were case-control in design, and one was a cohort study.11 The studies recruited patients from 20 countries, of which seven studies were from China, three from the USA, three from Japan, two from Israel, and two from Iran. The median year of publication for all articles analysed was 2016, illustrating a recent increase in the evidence for and interest in this subject.
Of the studies included, 21 of the 31 had methods enabling the detection of a vast number of bacterial genera/species, with the other 10 having a more focused approach.
Quality assessment
The table summarising the outcome of all CASP checklists can be found in Supplement 2. Three articles were excluded at the quality assessment stage, and the reasons for their exclusion are shown in [/hl]Figure 1.[/hl]
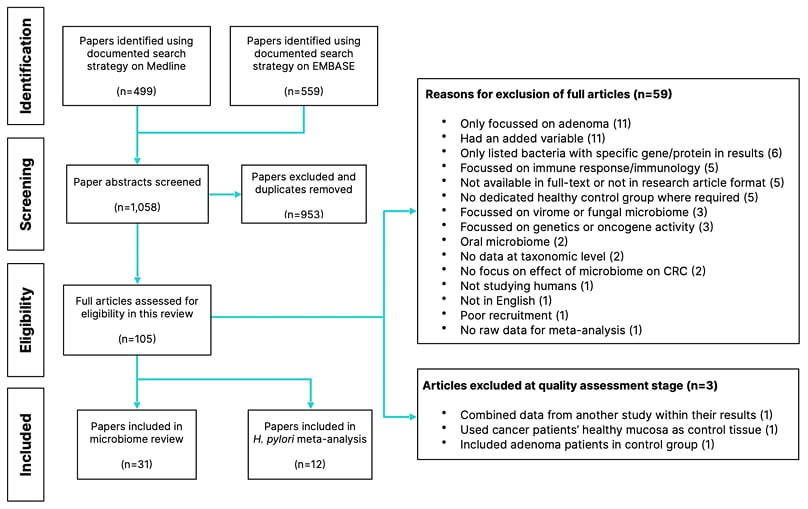
Figure 1: Flowchart showing the process and numbers of papers included and excluded at each stage.
CRC: colorectal cancer.
Significantly enriched genera in colorectal cancer and controls
Of all the taxonomic levels in the included papers, genus was most widely reported as being significantly raised in either group. Therefore, it was decided that genus and species level should be predominantly reported to provide more specific results. Significance was determined from the p-value tests used in each paper. Any species or genus whose p-value was <0.05 in either group were recorded. The full data extraction table can be found in Supplement 3.11-41
A total of 76 genera were either recorded as being significant, or as including a species within that genus that was independently significant. When compared, 58 of those genera were recorded as enriched in patients with CRC in at least one study, compared with 35 in controls. The frequency with which a genus or species was significant is recorded in Table 1.
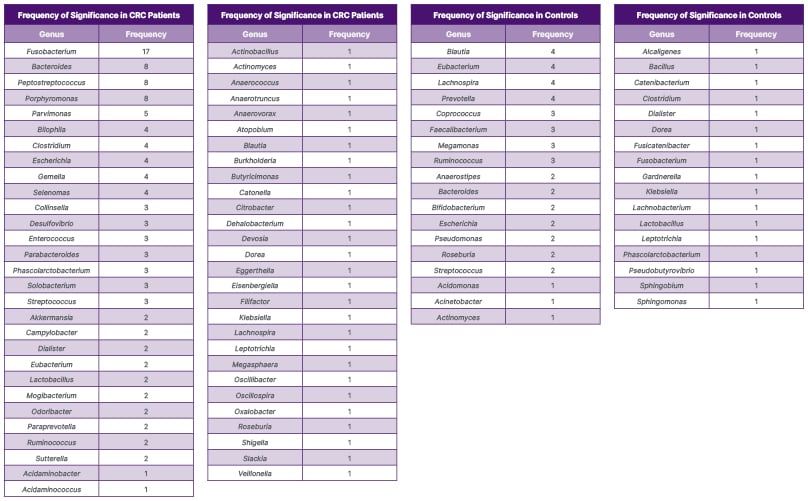
Table 1: Number of articles in which a genus or species within a genus was statistically enriched.
CRC: colorectal cancer.
Fusobacterium, or a species within this genus, was recorded as statistically enriched in patients with CRC in 70.8% of all studies powered to detect it. Within these studies, the whole genus was significantly enriched in a total of 11 papers, with individual Fusobacterium species enriched in nine of these papers (one of which was in a control group). Peptostreptococcus and Porphyromonas were raised in 40% of the eligible studies, Bacteroides was raised in 33.3% of the eligible studies, and Parvimonas was raised in 25% of the eligible studies. The median frequency of significance for all genera significantly enriched in the CRC group was one (interquartile range [IQR]: 2–16).
In contrast, Blautia, Eubacterium, Lachnospira, and Prevotella were significant in 20% of eligible papers in control groups. The median frequency of significance within the control groups was 1 (IQR: 1–3).
Significantly enriched species in colorectal cancer and controls
Of the Fusobacterium genus, F. nucleatum was the most commonly raised species amongst patients with CRC in the papers analysed, and was statistically enriched in seven papers. Only two individual species were identified as significantly enriched in this group, F. nucleatum and F. varium, with the latter only enriched in a single study.16 A third species, F. peridonticum, was recorded as significantly enriched in controls once.16
Bacteroides fragilis was significantly enriched among patients with CRC in five studies. Two of these papers looked for this species specifically. A total of eight other Bacteroides species were recorded as enriched in CRC compared with four in controls, however, each species was only significant once. A summary of the species reported as significantly enriched in two or more papers is provided in Supplement 4.
Both Enterococcus faecalis and Escherichia coli were significantly enriched in patients with CRC in three studies. However, two of these came from papers that were looking specifically for those bacteria.
Streptococcus bovis was specifically tested for in three studies, although only one of these studies returned a statistically significant enrichment in patients with CRC compared to controls.
Meta-analysis
The meta-analysis in this article exclusively looked at the association between H. pylori infection and the risk of CRC. A total of 12 papers, including seven case-control, two cohort and three cross-sectional, were identified from the search strategy and eligibility criteria used for this review. The meta-analysis included data of 20,001 events, out of a total of 64,027 participants.
The Forest plot (Figure 2)42-53 is split by study design for transparency with an overall OR. A full table of the study characteristics of the papers included in the meta-analysis can be found in Supplement 5.42-53
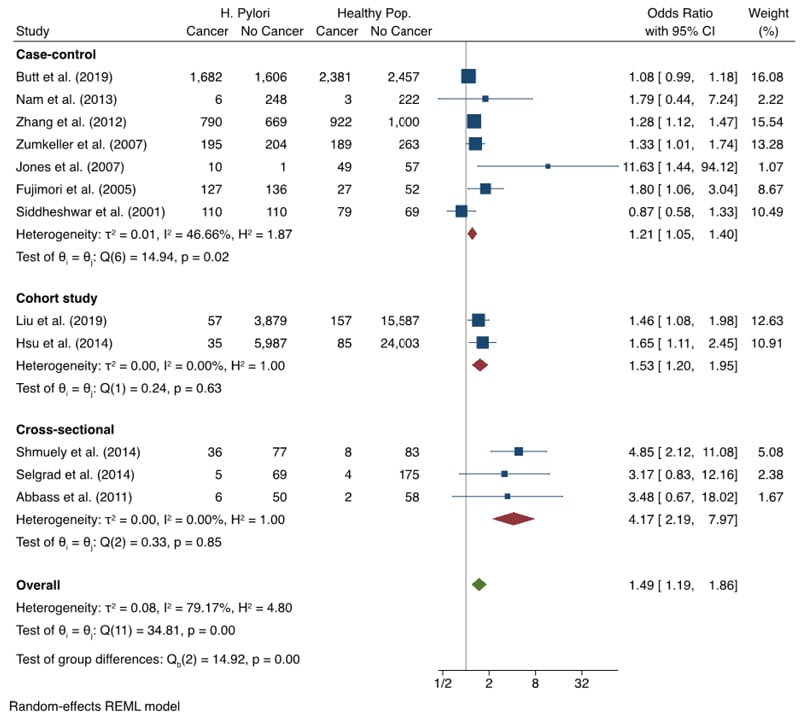
Figure 2: Forest Plot showing meta-analysis on association between Helicobacter pylori infection and colorectal cancer.42-53
This meta-analysis found an OR of 1.49 (95% CI: 1.19–1.86), showing a statistically significant link between H. pylori infection and increased risk of CRC (Figure 2).42-53 Upon exploration of publication bias, significant risk was found. Funnel plots proved to be asymmetrical, and Egger’s test gave a p-value of 0.0001, showing evidence of bias. When corrected for using the trim-and-fill method, the calculated OR came to 1.26 (95% CI: 0.93–1.71).
An I2 result of 79.17% showed substantial heterogeneity between studies, largely arising from the case-control group. The sub-analysis by study design showed a statistically significant link with each design. The largest effect was seen amongst cross-sectional studies.
DISCUSSION
Review
Results
Some bacterial genera were featured amongst studies at a higher frequency than others. Fusobacterium was documented most frequently (n=17), and was reported as significantly enriched in patients with CRC more than twice as often as Bacteroides, Peptostreptococcus, and Porphyromonas, which were the next most commonly featured genera (n=8). Conversely, the most frequently featured genera in the control group were only significant in four studies, with 16 of the 31 studies reporting any significant enrichment in this group.
Table 1 illustrates the high disparity in the reporting of taxa significantly associated with CRC and controls, amongst the included studies. Of the 58 significant genera identified in patients with CRC, 30 of these were significant in only one paper. Similarly, 20 of the 35 significant genera identified in the controls were associated in one paper.
Additional affiliations can be seen at the species level, as 57 different species were found to be significant in patients with CRC in at least one study. The most featured of these was F. nucleatum (n=7), followed by B. fragilis (n=5; seven if including enterotoxigenic strains). The frequency of replication of these results, however, was low. Only 13 of the 57 species were featured more than once between papers, as can be seen in Supplement 4. This replication frequency was even lower for species enriched in controls, with only three of the 40 species across articles being replicated more than once.
Limitations of included studies
Recruitment methods were a limitation of many studies included in this review. Controls in most of these studies were not recruited effectively. Most studies recruited from colonoscopy waiting lists, meaning that controls were presenting with colorectal symptoms warranting further investigation. Thus, the conclusion cannot be drawn that these are truly ‘healthy’ controls, and the control microbiome may have been altered as a result of the symptoms. Cases, however, were often recruited in an acceptable way, predominantly due to their diagnosis.
Papers also failed to consider the presence or absence of blood in the stools of the patients being studied. A recent article by Chenard et al.54 showed significant differences between the gut microbiome of those with and without blood present in their stools. Specifically, Bacteroides uniformis, Clostridium symbiosum, and Collinsella aerofaciens were found to be significantly enriched in participants with bloody stools. All of these species were found to be significantly enriched in patients with CRC in this review. The Bacteroides genus was also significantly enriched in this group. Conversely, Faecalibacterium prausnitzii, Prevotella copri, and Roseburia faecis were enriched in the controls described by Chenard et al.,54 as well as the controls in this review. As blood in the stool is considered a key sign of CRC,55 this could explain some of the disparities between the microbiome profiles of patients with CRC and healthy individuals.
Many articles included in this review hypothesised that the microbiome has a causative role in CRC carcinogenesis. Causation, however, cannot be inferred through the study designs adopted. Case-control designs, as adopted by all but one study, cannot determine whether CRC is caused by the microbiome profile detected, or whether this profile arises due to environmental changes caused by CRC, due to testing at a set-point as opposed to testing over time. It is of no surprise that a case-control design was used in the majority of studies, as a causal relationship can often be inferred using these methods. However, in the case of studying the potential role of the microbiome in carcinogenesis, many factors may increase or decrease the abundance of different bacterial species at a set point in time. Therefore, concluding that the enrichments are purely as a result of their involvement in CRC carcinogenesis risks oversimplifying a complex array of interactions.
Microbiome sampling also differed between studies. Included in this review, Chen et al.39 performed a mix of rectal swabs, faecal sampling, and biopsy for microbiome detection, and found significant differences between each. Therefore, a truly representative microbiome profile may not have been obtained in many of the studies that used only one detection method.
There were inconsistencies in the reporting of characteristics such as sex distribution, tumour staging, and sequencing platforms used, which would have been useful in the meaningful interpretation of these results.
The studies included in this review did not routinely report on the medication use of participants. Research by Vich Vila et al.56 has shown evidence for extensive changes in the gut microbiome composition with commonly used medications such as proton pump inhibitors, metformin, and laxatives. This interaction has the potential to further impact the risk of CRC development, and thus, requires further research.
Limitations of this review
The research included in this review originated from a wide variety of countries and the potential difference in microbiome related to diet was not thoroughly evaluated. Papers have found differences in the microbiome between patients with varying diets,57,58 which could predispose participants with certain diets to already high levels of specific bacteria, as well as a higher diversity of bacteria.59 Thus, this disparity may, in part, be due to the variations in diet between cultures.
A growing number of studies have emphasised how host genetic variations influence the gut microbial phenotype and how these multivariable interactions contribute to the development of CRC.60,61 While host genetics were outside the scope of this review, the authors recognise its potential relevance and importance as an increasing area of interest.
The focus of this article on genus and species taxonomic levels may also be seen as overly specific, when a more thorough analysis of all taxonomic levels implicated in CRC may provide a broader consensus. As this review excluded studies not written in English, due to language limitations; grey literature; and print-only journals, this decision may have excluded papers that could have enlightened further on this subject. The authors also acknowledge that more databases could have been searched as part of their research. The risk of bias amongst the included studies was also not thoroughly explored with the use of formal, published tools.
Meta-analysis
Results
As can be seen in Figure 2,42-53 the OR of 1.49 (95% CI: 1.19–1.86) shows a statistically significant link between H. pylori infection and CRC. However, this meta-analysis found considerable heterogeneity, which must be considered when inferring clinical significance. As part of the analysis, a funnel plot (Supplement 6), contour-enhanced funnel plot, Egger’s test, and the trim-and-fill method were run on the extracted data to search for evidence of publication bias.
The main sources of heterogeneity for this meta-analysis are likely due to population size differences and disparities in detection methodology. Recruitment study design also evidently contributed to the I2 statistic, shown by the moderate-to-low heterogeneity within each design’s subgroup-analysis (Figure 2).42-53
This result, when taken without correction for potential publication bias, shows a considerably raised risk when compared with other known CRC risk factors. With red meat consumption at a risk ratio of 1.12 (95% CI: 1.03–1.21),62 and obesity at a risk ratior of 1.19 (95% CI: 1.11–1.29),63 H. pylori infection proves to be a significant risk for CRC carcinogenesis.
The analyses discussed in this article showed evidence of publication bias. After running the trim-and-fill method, it was calculated that the addition of the five studies theoretically missing from the results would have resulted in a statistically insignificant link between H. pylori infection and CRC. Without these studies however, it is impossible to tell whether this is correct. Therefore, the result as it stands indicates that infection with H. pylori significantly increases the risk of CRC.
Limitations of included studies
There was a variation in the methods used to detect H. pylori infection. Many used detection of IgG through ELISA, others used tests such as urease and carbon breath tests. Using IgG detection, it is impossible to work out whether the infection is active or already eradicated. Both historic and ongoing infection may increase CRC risk, however, for this hypothesis to be tested effectively, infection must be confirmed as either active or eradicated alongside IgG detection.
Limitations of this meta-analysis
As the initial aim of the paper was not to perform a meta-analysis on this subject, this search strategy was not fully comprehensive. An extra search into H. pylori alone may have returned more papers and provided additional data for the analysis.
The large degree of heterogeneity may be a limitation, potentially affecting the clinical significance of this meta-analysis and its results. Meta-regression was also not performed by the authors, which could have further investigated this heterogeneity.
Future Research
As discussed, the case-control design is not sufficient to determine causality in this instance. This paper has identified the most frequently replicated key genera and species linked to CRC. However, due to the limitations of these study designs, it cannot be concluded that these bacteria raise the risk of developing CRC. To study this effectively, a large population, prospective cohort study should be performed, with regular colonoscopy and microbiome sampling. This sampling should be a combination of faecal, swab, and biopsy sampling. This would facilitate the representative characterisation of the microbiome profiles most commonly linked to CRC development, allowing a conclusion to be drawn as to whether there is any causative relationship between the human gut bacterial microbiome and CRC.
Implications of This Paper
To the authors’ knowledge, this is the first review of this type conducted in this topic. The results of this paper, combined with those of prospective cohort studies, as suggested, may have implications in the personalised management of patients to reduce their risk of CRC. The potential implications of the use of probiotics to lower risk has not yet been fully investigated and requires serious attention. However, this paper has identified species that may be of interest in a study of this nature.
It has been documented that probiotics could shape the intestinal microbiota.64 Knowledge of this, together with gut microbiome profiles associated with CRC, creates the potential for prophylactic probiotic administration to create a gut microbiome with lower CRC risk. Previous studies have alluded to the effectiveness of probiotics in the treatment and prevention of CRC.65-67 If CRC-associated bacteria can be significantly reduced within the microbiome, and healthy-associated bacteria increased through the use of probiotics, for example, this may reduce an individual’s risk of developing CRC.
CONCLUSION
This narrative review demonstrates the paucity and discrepancies amongst current research into the elevated risk bacteria pose on CRC development. However, considering these limitations, certain consistencies were present within the data extracted. There is a considerable link between the Fusobacterium genus and CRC, with a possible link suggested between bacterial presence of F. nucleatum and B. fragilis and increased CRC risk. Additionally, the meta-analysis suggested a significant association between H. pylori presence and CRC risk. However, both the narrative review and the meta-analysis require further expansion in methodology, as well as an improvement in research papers looking to elucidate a more significant association. This assessment finds the need for a large-scale cohort study over a significant period of time instead of a case-control study, in order to elucidate a potential carcinogenic relationship. This review has documented all bacterial genera and species that were significantly enriched in patients with CRC or controls in eligible papers. The hope is that this will help guide future research into the role of the microbiome in CRC carcinogenesis, with the goal of more personalised management of CRC case prevention for patients in the future.







