Abstract
Clostridioides difficile infection (CDI) presents a major global healthcare challenge. Recurrent/refractory disease is particularly hard to manage, and novel therapeutic strategies have recently been adopted. In particular, within the past decade, faecal microbiota transplant (FMT) has rapidly progressed from a ‘potential’ treatment option of fringe interest to one of the global mainstays of therapy for recurrent/refractory CDI. The first randomised study of its use for this indication was published as recently as 2013, but the emergence of subsequent randomised studies has led to its rapid adoption into guidelines and treatment algorithms. Very rare but serious reports of infection transmission from donor to recipient have resulted in ongoing refinements to donor screening, including the adoption of routine screening for intestinal carriage of multidrug resistant bacteria and severe acute respiratory syndrome coronavirus 2 status. Developments in the evidence base have given new insights into optimal recipient selection and preparation. Upper and lower gastrointestinal administration of FMT slurry are safe and effective in treating recurrent or refractory CDI, although the newer option of capsulised FMT has recently grown in popularity. The ‘next generation’ FMT products of defined microbial communities derived from donor stool are in late phase clinical trials and may become licensed for use in the near future. While different regulatory structures for FMT use have been adopted in different countries, the development of international networks of FMT-interested specialists has helped to harmonise best practice.
INTRODUCTION
Clostriodioides difficile infection (CDI) remains globally one of the major causes of hospital-acquired infection,1 with almost half a million cases occurring annually in the USA alone.2 Over the past two decades, several interrelated global changes in the pattern of CDI have made it particularly challenging to treat, including rising rates of metronidazole failure,3 the emergence of hypervirulent strains (particularly B1/NAP1/027),4 and rising rates of CDI recurrence. Specifically, the risk of recurrence within 8 weeks following treatment for primary CDI is up to 25% and rises as high as 65% for patients experiencing further recurrences.5
As such, there has been a major need for the development of novel therapeutic approaches to the condition, particularly for recurrent or refractory CDI (rCDI). While a vancomycin taper has been a well-established standard of care for this, there is also now evidence for the use of fidaxomicin (a novel macrocyclic antibiotic)6 and bezlotoxumab (an anti-toxin B monoclonal antibody),7 both of which reduce recurrence risk compared with vancomycin. However, neither of these treatments completely fill the therapeutic gap. For instance, concerns exist with fidaxomicin regarding its expense, limited evidence in treating CDI with severe colitis, and apparent limited efficacy in treating B1/NAP1/027 disease.6
Antibiotic use is well-established as the major risk factor for CDI, with antibiotic-mediated perturbation of the gut microbiome facilitating the colonisation of the distal gut by C. difficile, from which it can undergo growth, toxin production, and cause disease.4 If such disruption of the gut microbiome precipitates CDI, then restitution of the microbiome back to pre-morbid composition and functionality is an attractive therapeutic strategy. The first randomised trial investigating the use of faecal microbiota transplant (FMT) in the treatment of rCDI was reported in 2013, comparing rates of disease resolution in patients treated with fresh FMT administered via nasoduodenal tube compared with those receiving either vancomycin alone or vancomycin and bowel lavage.8 This trial was stopped prematurely on ethical grounds as rates of resolution at an interim analysis were significantly higher in those in the FMT arm than those in the vancomycin arms; reported side effects consisted principally of self-resolving gastrointestinal (GI) or systemic symptoms.8 Other randomised studies that quickly followed demonstrated similarly impressive safety and efficacy profiles when donor FMT was used to treat rCDI via nasogastric tube,9 enema,10 and colonoscopy.11,12 Subsequent studies demonstrated improved efficacy rates when healthy donor FMT was used compared with ‘autologous’ FMT,13 and that FMT produced higher remission rates than vancomycin.14 Systematic review and meta-analysis has been helpful for collating the published clinical data on FMT for rCDI, with a recent study estimating a number needed to treat compared with vancomycin of 2.9 for a single FMT and 1.5 for repeat FMT.15
In the UK, these randomised trials and other supportive studies have led to the adoption of FMT as a recognised treatment for rCDI in guidelines from Public Health England (PHE),16 the National Institute for Health and Care Excellence (NICE),17,18 and joint guidelines from the British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS).19,20 European, American, and other international consensus guidelines have also been published.21-23
The authors present an overview of current best practice in the use of FMT for the treatment of rCDI.
FAECAL MICROBIOTA TRANSPLANT DONOR RECRUITMENT AND SCREENING
The recruitment and retention of donors of healthy stool is central to a safe and effective FMT pathway. A recent multicentre study found that both social norms and logistics may be significant barriers to donation.24 Education on the benefits of FMT to others has been shown to encourage donation. Remuneration is also a motivating factor, a practice that is common in certain settings (including North America) but not currently in the UK and Europe.24,25
Potential donors are initially screened via either interview or a questionnaire, which covers basic demographic information. If they remain eligible, the next step is a more detailed medical assessment, looking at personal and family history in particular; this focuses on the wide range of both GI and non-GI conditions related to the gut microbiome, as well as risk factors for transmissible diseases (e.g., risk factors for blood-borne viruses). In general, donors should be between 18–60 years old and have a BMI within the healthy range; however, the recent use of antibiotics (typically within 3 months) is a common reason for exclusion.20 There is also a low threshold for exclusion of potential donors with a history of medical conditions clearly related to the gut microbiome, such as inflammatory bowel disease (IBD). Furthermore, given the association between a growing number of non-GI diseases (including metabolic, rheumatological, and neurological conditions) and perturbation of the gut microbiota, potential donors with a history of any such conditions are also excluded.22 Further health questionnaires are normally used at the point of each donation (e.g., regarding recent acute illness or travel to areas with endemic GI infection that may contraindicate donation).
There has been some debate about the use of related versus unrelated donors. While it is generally accepted that both may be safe and effective,26,27 most guidelines suggest that the use of a healthy, unrelated donor is preferable.
A significant risk related to FMT is the transmission of potential pathogens from donor to recipient. There are strict guidelines on laboratory screening of potential donors, though these may vary between regions (Table 1).20 With certain blood tests, such as Epstein-Barr virus and cytomegalovirus serology, some authorities recommend that these are only strongly indicated if the likely recipient is immunocompromised.20
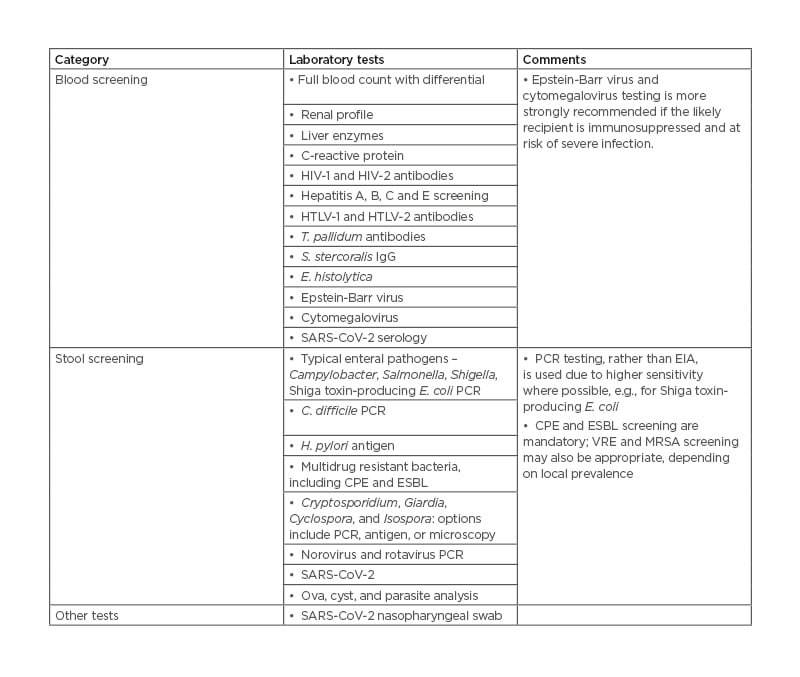
Table 1: Laboratory screening protocol for faecal microbiota transplant donors.20,22,23,24
Adapted from previously published guidelines.
C. difficile: Clostridioides difficile; CPE: carbapenemase-producing Enterobacteriales; E. coli: Escherichia coli; ESBL: extended-spectrum β-producing lactamase; E. histolytica: Entamoeba histolytica; EIA: enzyme immunoassay; H. pylori: Helicobacter pylori; HTLV: human T-lymphotropic virus-1; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; MRSA: methicillin-resistant Staphylococcus aureus; S. stercoralis: Strongyloides stercoralis; T. pallidum: Treponema pallidum; VRE: vancomycin-resistant Enterococci.
The frequency of re-testing potential donors depends on the FMT method. As discussed below, the use of banked frozen samples over fresh samples is now strongly recommended in most territories, in part due to the need for fewer and less frequent donor screenings, increasing convenience for donors and reducing cost for centres. With frozen FMT, donors will typically donate regularly for a defined period of time, with health questionnaires and full serology and stool screening at the start, and repeated at the end, of the donation period (‘bookending’). FMT prepared during these periods of screening is held in ‘quarantine’ until both screens are clear and the FMT can be safely released for clinical use. In centres still using fresh FMT, regular donor laboratory screening (with a further health questionnaire at the time of each donation) has been suggested; however, this clearly has an inferior safety profile compared with frozen FMT, as the material is likely to be administered before the extensive laboratory screen can be completed.
A number of clinical reports of FMT-related transmission of infection have been described, which have resulted in adaptation and modification of FMT donor screening protocols.28 There have been recent concerns about FMT-related transmission of multidrug resistant bacteria, with two cases of extended-spectrum β lactamase (ESBL)-producing Escherichia coli bacteraemia occurring in separate clinical trials, albeit both from the same stool donor.29 One of these cases was fatal. At the time of donation, screening for ESBL-producing organisms was not mandated by the U.S. Food and Drug Administration (FDA), although screening for these (as well as other multidrug resistant bacteria) was subsequently advised.30 Consistent with this, guidelines produced since 2018 have recommend ESBL and carbapenemase-producing Enterobacteriales CPE stool screening universally.20 In 2020, four cases of Shiga toxin-producing E. coli infections were reported in the USA, again from a single donor.31 The stool had been screened for Shiga toxin-producing E. coli with enzyme immunoassay, with a negative result, but was later found to be positive on nucleic acid amplification testing (NAAT), a more sensitive method.32 The FDA now mandates NAAT for future screening, and BSG and HIS guidelines already specify the use of PCR, a form of NAAT.20
Furthermore, the COVID-19 pandemic has introduced new challenges to donor recruitment and screening. Collection of FMT donor samples was postponed in many regions at the height of the pandemic, with the ‘shelf life’ of previously frozen samples being extended to enable continued treatment. As FMT has been deemed a vital procedure, rapid updates and adjustments to guidelines have been made to adapt to the pandemic.33,34 Risk assessment has been updated to assess for exposure to COVID-19, and nasopharyngeal swabbing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was also recommended.33 However, recognising that SARS-CoV-2 may be detectable in stool,35 guidelines were updated further, to strongly recommend that molecular stool testing should be carried out where possible. There is some evidence that this may be the best way of reducing the risk of transmission,36 and validated assays have recently been approved,37 facilitating the resumption of FMT services. The response to the COVID-19 vaccination programme is not yet entirely clear, but as the current validated vaccines do not use a live attenuated virus, it has been suggested there is no risk of transmission from vaccinated donors.38
WHEN TO CHOOSE FAECAL MICROBIOTA TRANSPLANT AS A TREATMENT FOR CLOSTRIODIOIDES DIFFICILE INFECTION
Despite some small studies investigating the use of FMT as a treatment for primary episodes of CDI,39,40 it is widely accepted that antimicrobial therapy remains the mainstay of treatment in this scenario.
As outlined above, current guidelines recommend that the major indication for FMT is in recurrent or refractory CDI, provided that there has already been previous treatment with ‘standard of care’ therapies (i.e., vancomycin or fidaxomicin).20,22 There are no data definitively stating how many recurrences after treatment with antimicrobials are required before FMT merits consideration in CDI, but UK guidelines recommend considering FMT after two recurrences or one recurrence with risk factors for further episodes.20 While there is no uniform definition of success or failure after FMT for rCDI, published guidelines have strongly recommended that repeat FMTs are indicated where a single FMT alone does not cause disease remission.20,22
There are few absolute contraindications to FMT, although anaphylaxis or severe allergic food allergy is often included in this list. One option in this scenario may be patient-directed selection of a stool donor on a diet avoiding any potential food allergens; similarly, a donor on a gluten-free diet may be appropriate for a recipient with coeliac disease.20 Pregnancy and lactation may be viewed as relative contraindications. While earlier small retrospective studies had suggested a risk of an IBD flare when FMT was administered to patients with IBD and super-added CDI, a recent prospective study of FMT in this scenario did not corroborate this.41,42 Despite initial concerns about bacterial translocation and risk of sepsis when FMT was administered to patients with cirrhosis and CDI, more recent data demonstrate that it is safe and effective in this setting.43 There appears to be no additional risk associated with the use of FMT to treat rCDI when administered to patients who are immunocompromised.44,45
The largest study reported on FMT in children to date is from a multicentre retrospective cohort study of 372 patients receiving FMT for CDI.46 CDI resolution after one or two FMTs was >80%, and adverse events were, overall, comparably modest to those occurring in adults who receive FMT. In a joint position paper from North American and European paediatric gastroenterologists, the use of FMT was recommended in children with CDI for similar indications to those in adults.47
ROUTES OF FAECAL MICROBIOTA TRANSPLANT ADMINISTRATION
Conventionally, FMT administration routes were principally categorised into upper GI (nasoduodenal/nasogastric tube, or gastroscopy) or lower GI (colonoscopy, flexible sigmoidoscopy, or enema). Systematic review and meta-analysis has demonstrated that enema appears to be the least efficacious route for a single administration; lower GI and upper GI administration appear to be of comparable efficacy, with colonoscopy being the single most effective route of administration.15 The potential discrepancy in efficacy between upper and lower GI routes is less relevant in the context of multiple infusions, where efficacy rates of different routes are comparable.15 Other considerations for a preferred route of administration may be practical. For instance, colonoscopy may be desirable in particular circumstances for allowing endoscopic assessment of the large bowel, while nasogastric tube may be more pragmatic for patients who are older and frailer, and who may not tolerate endoscopic procedures.
An alternative route of delivery to these conventional methods is capsulised FMT, whereby the faecal matter is delivered via oral capsules; this can either be in the form of capsulised frozen slurry or as lyophilised material. In the largest randomised controlled trial exploring capsulised FMT in the treatment of rCDI to date, capsules demonstrated similar efficacy to colonoscopy in terms of successful prevention of rCDI (>95%) in both patient groups.48 Capsule administration eliminates the need for invasive procedures and potential complications secondary to these. However, different centres using capsules have prepared them using different methodologies, and a ‘dose finding’ exercise might be required to find the balance between a threshold number of capsules to successfully treat most cases of CDI versus an acceptable capsule burden to ingest.
Depending on which route is used, patient preparation varies prior to the procedure. Irrespective of route of delivery, a further course of anti-CDI antibiotics (with a washout period just prior to FMT administration) is recommended. Bowel lavage (e.g., with polyethylene glycol) may help to reduce C. difficile burden further and remove residual antimicrobials. For upper GI administration, many centres recommended proton pump inhibitors and pro-kinetics prior to administration.20 Anti-motility drugs (i.e., loperamide) may be considered after lower GI administration to aid retention.
STOOL BANKING AND REGULATION
An evolution in FMT protocols has been the widespread use of frozen faecal material that can be prepared from screened donors in advance of a planned FMT and thawed, transported, and administered when treatment is required (commonly using glycerol as cryopreservative). A non-inferiority randomised controlled trial demonstrated no significant difference in safety or in efficacy between fresh and frozen FMT, and frozen FMT10 confers a number of logistical advantages as discussed above. This has resulted in a trend towards a shift from FMT services operating as small, local centres towards centralised stool banks, where expertise, traceability, and standardised procedures translates into increased safety and quality control of the production.49 This ultimately has allowed the development of ‘hub and spoke’ FMT network arrangements, allowing FMT treatment to be available at centres that would otherwise have been limited due to lack of facilities and resources.22
The development of stool banks has also been helpful from the perspective of developing standardised pathways for co-ordinating an FMT service, from which clinical experience can be shared between interested parties internationally. However, challenges still remain with regard to aspects related to FMT regulation and governance of FMT services. In the UK, FMT is regulated as an unlicensed medicinal product by the Medicines and Healthcare Products Regulatory Agency (MHRA), and best practice regarding manufacturing, production quality control, and donor screening governance has been defined in national guidelines.20 In contrast, in other countries, FMT has been regulated as a tissue or transplant material.22,23 Within certain regions, national FMT registries have been established, providing a useful tool for audit and research.50,51
The largest stool bank globally has been OpenBiome, based in Boston, Massachusetts, USA. Recently, the large stool bank in Birmingham, UK (Microbiome Treatment Centre), published their FMT methodology, which received licensing in accordance with the MHRA guidelines for the production and distribution of FMT as a medicinal product. This has been fundamental in extending the reach of this treatment within the UK National Health Service (NHS), as well as providing a validated framework for implementation across other countries.52
OUTSTANDING ISSUES, NEXT STEPS, AND CONCLUSIONS
Despite the clear efficacy of FMT in the treatment of rCDI, there are several remaining uncertainties related to its use. For example, concerns have been raised about the relatively small size of randomised trials published and limited follow-up before FMT reached widespread adoption, especially when this therapy lacks standardised dosing or formulation and well-defined mechanism of action, which are required for the introduction of other therapeutics.53 The emergence of longer-term patient follow-up data after FMT for rCDI has helped to alleviate some of these concerns.54 There remain gaps in knowledge related to mechanism of action, although progress has been made in this area too (Figure 1).55,56 There is also a theoretical concern about gut microbiota ‘traits’ being transmitted from donor to recipient (e.g., an increased risk of developing IBD in the future), although there has been no conclusive demonstration of such an occurrence.
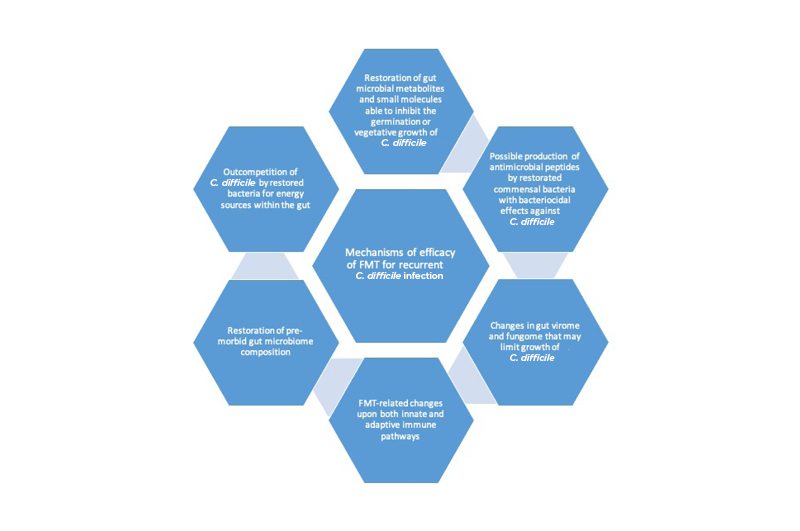
Figure 1: Mechanisms of efficacy of faecal micriobiota transplant in the treatment of recurrent Clostridioides difficile infection.
C. difficile: Clostridioides difficile; FMT: faecal microbiota transplant.
While the development of capsulised FMT has helped to avoid some of the drawbacks associated with FMT use (e.g., invasive administration of slurry), it does not avoid all of the drawbacks. There is considerable interest in ‘microbial therapeutics’ and ‘next generation’ FMT products. Recently, there have been reports of initial results of two microbiome-based therapeutic products used in clinical trials for rCDI, including a Phase III study of a spore-based therapy (SER-109) undertaken by Seres Therapeutics (ECOSPOR III study; Cambridge Massachusetts, USA), and a Phase II study of a ‘whole microbiome’ investigational product from Finch Therapeutics (CP101; PRISM3 trial; Somerville, Massachusetts, USA).57 Both products met efficacy endpoints. Should these products reach clinical endpoints in larger studies, it seems likely that there will be a strong case for consideration of their licensing, which may require further evaluation of their cost, accessibility, and other issues.58
Another challenge for FMT more generally is its use beyond the remit of rCDI. Given the increasing number of medical conditions associated with perturbation of the gut microbiome, there is great enthusiasm for trialling FMT in the management of a range of different conditions.55 While there are signals of clinical interest for the use of FMT for non-CDI indications (including for the induction of remission in mild to moderate ulcerative colitis, or transient improvement in insulin sensitivity in metabolic syndrome),28,55 there has not been a comparable level of durable clinical benefit observed as that seen in rCDI. As understanding of the contribution of the gut microbiome to these conditions expands, there may be an opportunity for more nuanced application of FMT or other microbial therapeutics, taking into consideration donor and recipient factors in more detail.59








