Abstract
A 54-year-old male with a history of multiple autoimmune arthritides was admitted following a 3-week history of progressive dysphagia with odynophagia to solids and liquids, with significant weight loss, night sweats, and exertional dyspnoea. Oesophagogastroduodenoscopy revealed an obstructing oesophageal stricture. Blood tests showed neutropaenia and high levels of inflammatory markers, suggestive of primary oesophageal malignancy. Oesophageal and bone marrow biopsies demonstrated inflammatory change not suggestive of malignancy. PET showed highly active nodules in the left lung and sigmoid colon, but the oesophagus was clear. Following a clinical rheumatology review, a differential diagnosis of inflammatory lesions, most likely secondary to systemic rheumatoid, was considered. The patient responded well to high-dose intravenous steroid therapy. Subsequent outpatient interval high-resolution CT demonstrated complete resolution of the lung nodule. He was maintained on oral prednisolone and methotrexate, having no further symptoms of dysphagia or neutropenia. A literature search revealed no published reports or case studies outlining a similar history to the reported patient: rheumatoid arthritis presenting to hospital as potential oesophageal malignancy.
INTRODUCTION
A 54-year-old male with a history of multiple autoimmune arthritides was admitted following a 3-week history of progressive dysphagia with odynophagia to solids and liquids, associated with significant weight loss, night sweats, and exertional dyspnoea. Oesophagogastroduodenoscopy (OGD) revealed a malignant-looking stricture, thus promoting a working diagnosis of oesophageal malignancy.
Subsequent histological analysis from biopsies of the lesion demonstrated inflammatory change only, with no evidence of malignancy. The patient became repeatedly neutropenic; however, subsequent bone marrow biopsies and repeat oesophageal biopsies did not demonstrate malignant cells. PET-CT revealed an active lesion in the left lower lobe of the lung, but not the oesophagus. Following a clinical rheumatology review, a differential diagnosis of inflammatory lesions, most likely secondary to systemic rheumatoid, was considered. The patient’s symptoms responded well to high-dose intravenous steroid therapy. Subsequent outpatient interval high-resolution CT scans demonstrated complete resolution of the lung nodule. He was maintained on oral prednisolone and methotrexate, having no further symptoms of dysphagia or neutropaenia.
BACKGROUND
Rheumatoid arthritis is a chronic autoimmune symmetrical inflammatory condition of the joints, manifesting with progressive joint swelling and pain. It is associated with significant long-term morbidity and the necessity for lifelong medication (starting with nonsteroidal anti-inflammatories and ending with expensive and experimental monoclonal antibodies).1,2 These long-term medications are not without side effects. This case demonstrates the challenges in diagnosis when systemic arthritides mimic the classic, and far more common, presentation of malignancy.
Following OViD and PubMed literature searches, the authors found no published literature of similar cases.
This report highlights the atypical features of autoimmune inflammatory conditions which is important because raising awareness can help to avoid misdiagnosis of malignancy and the subsequent psychosocial implications such a diagnosis entails.
CASE PRESENTATION
A 54-year-old Caucasian male was admitted under gastroenterology following a 3-week history of dysphagia, odynophagia, weight loss, night sweats, and exertional dyspnoea. His medical history included strongly positive anticyclic citrullinated peptide rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis. He was prescribed 15 mg methotrexate once weekly, 5 mg folic acid once weekly, 60 mg acemetacin twice daily, 15 mg lansoprazole once daily, and 100 mg tramadol as required for pain. He reported noncompliance with medications (never taking acemetacin or methotrexate), and did not attend routine general practitioner c heck-ups or rheumatology clinics. He had a 30 pack-year smoking history, minimal alcohol intake, and an unremarkable travel, sexual, or family history. He described no skin changes.
INVESTIGATIONS
- Admission blood tests:
- A) Full blood count: neutrophils 0.52×109/L (1.80–7.50×109/L), white blood cells 2.50x 109/L (3.60–11.00×109/L), haemoglobin 124 g/L (130–180 g/L), mean cell volume 76.0 fL (80.0–100.0 fL), hypochromic red cell 12.9%.
- B) C-reactive protein 157 mg/L (<2 mg/L), erythrocyte sedimentation rate 67 mm/hour (<20 mm/hour).
- C) Albumin 19 g/dL (35–50 g/dL), liver function otherwise unremarkable.
- D) Urea 10.3 mmol/L (1.8–8.2 mmol/L), renal function otherwise unremarkable.
- E) HIV screen: negative.
- F) Rheumatoid arthritis investigations: strongly positive anticyclic citrullinated antibody 2,776.8 U/mL (<20 U/mL).
- OGD plus biopsy: likely malignant stricture seen at 27 cm from incisors, impeding progression of endoscope (Figure 1).
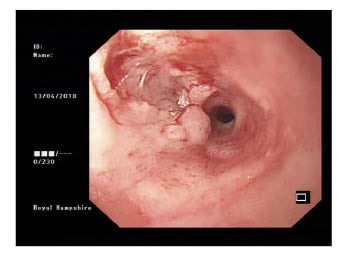
Figure 1: Oesophagogastroduodenoscopy appearances suggestive of malignant stricture.
- A) The oesophageal biopsy microscopic conclusion was that morphological and immunohistochemical appearances favoured an inflammatory process. Ulcerated and severely inflamed squamous mucosa, showing polypoid granulation tissue, Sheets of lymph plasmacytic cells expanded the lamina propria and obscured glands in places. The squamous epithelium that persisted showed atypia. No viral inclusions were seewwwn, and no epithelial malignancy presented. In view of lymphoplasmacytic cells, immunohistochemical staining was performed. Lymphoid population comprised of reactive B and T cells. There was no light chain restriction. Ki67 showed a low proliferation factor.
- CT chest/abdomen/pelvis: dilated oesophagus shown at the level of the carina with an air-fluid level. No obvious oesophageal wall thickening was apparent. Several subcarinal and para-aortic enlarged lymph nodes were revealed (≤1 cm).Lungs: panacinar emphysema. Left lower lobe subpleural inflammatory changes. Liver: no definite lesion.
Spleen: unremarkable, normal size.
- PET-CT: highly active speculated nodule in left lower lung lobe and sigmoid colon identified, concerning for malignancy. Diffuse low-grade activity along the oesophagus, presumably physiological, and no evidence of malignancy (Figure 2).
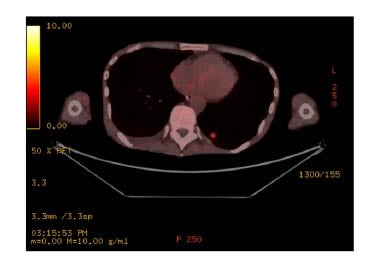
Figure 2: Left lower lung lesion as seen on PET.
- Bone marrow biopsy: inflammatory myelopathy only.
- Connective tissue disease screen including antinuclear antibodies, immunoglobulins (IgG, IgA, IgM), scleroderma antibody antitopoisomerase I antibody (SCL-70), antiribonucloprotein and centromere antibody: all unremarkable.
- Reviewed by haematology due to recurrent neutropaenia; differential diagnosis of lymphoma was considered and excluded based on oesophageal immunohistopathology and bone marrow findings.
TREATMENT
- Nasogastric feeding via endoscopically placed nasogastric tube.
- Initially high dose of intravenous dexamethasone (0.6 mg/kg) advised by rheumatologist to treat oesophageal swelling.
- Oesophageal dilatation for symptomatic relief.
- A) Repeat OGD biopsy post steroid therapy: lymphoblastocytoid cells and generalised oesophagitis. Consistent with inflammatory change.
- Maintenance therapy with prednisolone alone as methotrexate, azathioprine, and sulfasalazine contraindicated with neutropaenia.
DISCUSSION, OUTCOME, AND FOLLOW-UP
Throughout the course of his admission the patient had a number of potential diagnoses and was seen by four different subspecialty medical teams (gastroenterology, haematology, respiratory, and rheumatology). Primary oesophageal carcinoma was initially the working diagnosis, with a differential of lymphoma. Oesophageal and bone marrow biopsy showed inflammatory changes only. PET-CT further disputed oesophageal malignancy but raised the new possibility of lung malignancy. Following a lung multidisciplinary team meeting discussion, the lung nodule was initially considered to be an incidental lung primary carcinoma. Flexible sigmoidoscopy revealed no sinister lesion to corroborate the PET scan findings.
With all investigations taken into consideration, he was reviewed by a rheumatologist who diagnosed severe systemic manifestations of rheumatoid arthritis. Felty’s syndrome was excluded based on normal splenic appearances and size on cross-sectional imaging. His ‘hot-spots’ on PET-CT and the oesophageal stricture were considered inflammatory nodules, which increase the misdiagnosis of malignancy using thoracic PET-CT.3
The patient has been followed up in the rheumatology clinic and was maintained on prednisolone alone. At follow-up, he was experiencing Cushingoid side effects, but neutrophils and inflammatory markers were within normal limits and his symptoms of dysphagia and odynophagia had resolved entirely. Neutropenia was likely a result of systemic inflammation as noncompliance with methotrexate rules out this as a cause. He has not had any infections and was managed in the outpatient setting with a reducing course of prednisolone and omeprazole. High-resolution chest CT showed complete resolution of the suspicious lung nodule. Given his ongoing rheumatological joint pain, he awaits a haematological opinion to advise on further methotrexate or disease-modifying antirheumatic drugs management given his history of bone marrow suppression.
A literature search revealed no published reports or case studies outlining a similar history to this patient: rheumatoid arthritis presenting to hospital as potential oesophageal malignancy.
Oesophageal manifestations have been reported in Behcet’s disease.4 The patient had previously undergone investigation and Behcet’s had been excluded at the time of initial rheumatoid arthritis diagnosis. There are also reported cases of drug-associated oesophageal strictures (particularity nonsteroidal anti-inflammatory drug).5 However, these patients did not suffer complete dysphagia, but had significant odynophagia. In addition, there are no published cases of acemetacin-associated oesophageal strictures, which the patient was prescribed (and was not compliant with) making this a less likely diagnosis.
LEARNING POINTS: SHEDDING NEW LIGHT ON RARE PRESENTATIONS OF A COMMON CONDITION
Concerning clinical features suggestive of malignancy can be explained by other conditions, even if investigations are initially supportive of malignancy.
Oesophageal strictures and ‘hot spots’ on PET-CT can be associated with inflammatory conditions, such as rheumatoid arthritis.








