Meeting Summary
As the majority of patients with IgA nephropathy (IgAN) progress to kidney function loss, it is important to treat this primary glomerulonephritis in a way that will prevent or slow such progression. In a presentation delivered at the 2024 Nephro Update Europe congress, Jörg Latus, from Robert-Bosch-Krankenhaus GmbH, Stuttgart, Germany, discussed the relevance of assessing proteinuria levels in patients with IgAN and how this is recognised in the 2024 Kidney Disease Improving Global Outcome (KDIGO) guidelines, currently in draft form. Since the 2021 KDIGO guidelines, therapy choices for IgAN advances have widened and include those specifically developed for IgAN, such as the dual endothelin and angiotensin II receptor antagonist sparsentan and modified-release budesonide (MRB), as well as sodium-glucose cotransporter-2 inhibitors (SGLT2i), which have a broader role in chronic kidney disease treatment. Latus discussed some of the pivotal trials that led to these medications being approved for use in patients with IgAN, along with real-world data for sparsentan. Also discussed were trials for other IgAN pathogenesis-targeting medications, future approval of which is hoped to open the choice of treatments for patients with IgAN at risk of progressive kidney function loss such that more individualised therapy regimens can be provided.
Introduction
Although IgAN is a rare disease, it is a major cause of primary glomerulonephritis,1,2 and an important disease to recognise and control as the majority of patients with IgAN progress to kidney failure in the 20−30 years following diagnosis.3 In a presentation at the Nephro Update Europe congress, Jörg Latus, an expert in the field of kidney diseases, discussed IgAN treatment and highlighted the importance of recognising and controlling proteinuria as a means to mitigate kidney failure progression.4
In Europe, where the population is an estimated 745 million,5 the overall annual incidence of IgAN is 0.76 per 100,000, equating to around 5,662 new cases, with a point prevalence of 2.53 per 10,000, equating to around 188,485 patients. Point prevalence is highest in North-Eastern Europe and lowest in Southern Europe; it is also lower in paediatric and elderly populations.2 However, Latus said, “we all know that IgAN is underdiagnosed as a lot of patients are not biopsied”.
There are multiple factors involved in the pathogenesis of IgAN, as encompassed by the ‘four-hit’ model. Initiation of IgAN, prior to the first hit, occurs when stimulation of mucosal innate immune cells by the gut microbiome leads to increased activation of mucosal IgA-committed B cells via ‘B cell activation factor’ (BAFF) and ‘a proliferation-inducing ligand’ (APRIL) signalling. Hit 1 is then an increase in circulating levels of galactose-deficient IgA1 (gd-IgA1), with Hit 2 being the generation of anti-gd-IgA1 autoantibodies. Hit 3 is the formation of immune complexes containing gd-IgA1 and IgA, IgG, and IgM autoantibodies. This is followed by Hit 4, where there is glomerular mesangial deposition of these complexes and activation in mesangial cells of inflammatory and fibrotic pathways, including the complement system.6
According to 2021 KDIGO guidelines, idiopathic IgAN is diagnosed once secondary causes of IgA-dominant glomerulonephritis are ruled out. A kidney biopsy can then be assessed using the MEST-C instrument (mesangial [M] and endocapillary [E] hypercellularity, segmental sclerosis [S], interstitial fibrosis/tubular atrophy [T], and crescents [C]). The guidelines suggest that progression risk at diagnosis can be estimated using the International IgAN Prediction Tool, which includes estimated glomerular filtration rate (eGFR), blood pressure, age, and proteinuria at biopsy; race; renin-angiotensin system inhibitor (RASi) and immunosuppression use; and individual MEST-C component scores. Use of this tool can help inform discussion with the patient to lead to shared decisions regarding individualised treatment.7 However, observed Latus, it may not routinely be used in practice.
Much of this session discussed both the 20217 and the 2024 updated KDIGO guidelines, which are currently in draft form. It is to be noted that final guideline content may change based on feedback on the draft.8
The Role of Proteinuria in IgA Nephropathy
According to Latus, proteinuria levels are the “strongest prognostic factor for disease progression in IgAN”. Indeed, in clinical trials, proteinuria reduction is often used as a surrogate endpoint for therapy efficacy.9
Recently, the UK National Registry of Rare Kidney Diseases (RaDaR) carried out a study investigating proteinuria in patients with IgAN, including 30 years of data from 2,439 patients. Analysis showed that the median time to proteinuria from registry entry was 4.5 years, and that those with time-averaged proteinuria >0.88 g/g were likely to progress quicker to kidney failure or death compared with patients where this value was <0.88 g/g. Also shown was that an estimated 30% of patients with time-averaged proteinuria of 0.44 to <0.88 g/g, and 20% of patients where this figure was <0.44 g/g, developed kidney failure within 10 years of diagnosis.3 Similarly, a study analysing data from the Toronto Glomerulonephritis Registry (n=542) showed that survival without kidney failure was highest and longest in patients with proteinuria <0.3 g/day, and progression to kidney failure was highest and quickest in those with proteinuria >3.0 g/day.10
Even in patients with proteinuria in the low ranges, eGFR decline can be significant over a number of years.11 In the RaDaR study, for adults <50 years old at diagnosis, it was estimated that an annual change in eGFR of 1 mL/min/1.23 m2 would result in them reaching kidney failure in their expected lifetime. The RaDaR study also found that, although lowering proteinuria levels was associated with reductions in eGFR slope decline, the decline still occurred in patients even if there was no increase in proteinuria or if proteinuria levels decreased.3
Latus said that these studies show that “there is no safe proteinuria level”, overturning beliefs that he reported from colleagues that there is a low risk of kidney progression if proteinuria is <1.0 g/day. This is reflected in the updated 2024 KDIGO guidelines, which state that “a patient with IgAN is at risk of progressive loss of kidney function if they have proteinuria ≥0.5 g/d (or equivalent), while on or off treatment”.8
“This is a very important part of the guideline,” said Latus, and stressed how now “the 1.0 g/day should be deleted, and we must talk about 0.5 g/day”.
Treatments Targeting the ‘Four Hits’ of IgA Nephropathy
“In nephrology, we try to preserve kidney function,” explained Latus. This is reflected in the 2024 draft KDIGO guidelines, where the goal in patients with IgAN at risk of progressive kidney function loss “is to reduce the rate of loss of kidney function to <1 mL/min per year for the rest of the patient’s life”.8 However, commented Latus, “this is not that easy to achieve”.
In patients at risk of progressive kidney disease (proteinuria ≥0.5 g/day), management of nephron loss is driven by the need to tackle specific drivers and their response to treatment. This includes addressing IgAN from the perspective of an autoimmune disease and having treatment goals that include reducing pathogenic forms of IgA and IgA immune complex formation. Management also includes having treatment goals that include maintaining blood pressure control; reducing cardiovascular risk; reducing glomerular hyperfiltration and glomerular inflammation, and the impact of proteinuria on the tubulointerstitium.8
To achieve these goals, multiple drugs may be needed, Latus explained. The highest KDIGO recommendation is for a RASi such as an angiotensin-converting enzyme inhibitor (ACEi) or an angiotensin receptor blocker (ARB).7,8 While, according to Latus, RAS inhibition is “a significant pillar in the treatment of IgAN”, he also discussed a study investigating 96 patients prescribed a RASi, which showed that 3 months’ treatment led to complete proteinuria remission in only 6.3% of patients, with 30.2% showing partial remission and 63.5% having no remission.12
According to 2021 KDIGO guidelines, while glucocorticoids may also be added to therapy, prescribing should encompass individual risk stratification and only be considered in patients where eGFR is ≥30 mL/min/1.73 m2. Glucocorticoids should also be avoided in patients who have diabetes; a body mass index >30 kg/m2; latent infections, such as viral hepatitis or tuberculosis; secondary disease, such as cirrhosis; severe osteoporosis; uncontrolled psychiatric illness; or active peptic ulceration.13 The updated draft KDIGO guidelines also include patients with prediabetes and cataracts in those where glucocorticoids are not recommended.8 In his personal experience though, Latus reported how he treats very few patients with this medication.
Innovative Approaches to IgAN Treatment to Target Proteinuria
The updated KDIGO guidelines include treatments that are now approved for patients with IgAN,8 most notably sparsentan,14 SGLT2i,15 and MRB.16 Latus provided an overview of studies of these medications with regard to their actions on proteinuria.
Sparsentan
Sparsentan is a dual endothelin and angiotensin II receptor antagonist14 that the draft KDIGO guidelines recommend for patients at risk of progressive kidney function loss.8 In the international Phase III PROTECT trial, following discontinuation of maximal RASi, adult participants with 24-hour urine protein excretion (UPE) ≥1.0 g/day received either 400 mg/day sparsentan (n=202) or 300 mg/day of the ARB irbesartan (n=202), following a 2-week titration period where doses were 200 mg/day and 150 mg/day, respectively. Of note, highlighted Latus, in both groups, almost all participants (approximately 97%) received the full dose of each drug, with those not receiving such doses remaining on the titration period dose. The blinded treatment period was for 110 weeks, after which medication was discontinued. Standard care was then administered for 4 weeks, then participants from either group could take part in an open-label extension period of 400 mg/day sparsentan for 156 weeks.17,18
Mean, respective, baseline characteristics in the sparsentan/irbesartan groups were similar with regard to age (46.6 and 45.4 years) and gender (69% and 71% male), eGFR (56.8 and 57.1 mL/min/1.73 m2), and proteinuria (UPE 1.8 and 1.8 g/day; urine protein–creatinine ratio [UPCR] 1.3 and 1.2 g/g). They were also similar with regard to respective hypertension history (72% and 71%) and blood pressure readings (128.0/81.6 and 129.9/83.2 mmHg); time from kidney biopsy (4.0 and 4.0 years), and ACEi or ARB dose at screening (130 and 125 mg/day). No participant was treated with an SGLT2i.18
For the primary endpoint at 36 weeks, treatment with sparsentan resulted in a rapid, significant, and clinically meaningful greater reduction from baseline in proteinuria (–49.8%) compared with irbesartan (–15.1%). This represents a between-group relative reduction of 41% (least squares mean ratio: 0.59; 95% CI: 0.51, 0.69; p<0.001). In the sparsentan group, 31% had a complete response (UPE <0.3 g/day), compared with 11% in the irbesartan group (relative risk [RR]: 2.5; 95% CI: 1.6, 4.1), with partial response (UPE <1.0 g/day) shown in 78% of the sparsentan group compared with 53% of the irbesartan group (RR: 1.5; 95% CI: 1.1, 1.9). A post-hoc assessment showed the proportion of patients where UPE was <0.5 g/day to be 51% in the sparsentan group compared with 24% in the irbesartan group (RR: 2.1; 95% CI: 1.5, 2.9).18
This trial also examined changes in eGFR and showed that up to Week 110, a decrease in such was shown in both groups. The total slope (calculated from Day 1 to Week 110) and chronic slope (total slope minus the acute effect, calculated from Week 6–110) were, respectively, –2.9 and –2.7 mL/min/ 1.73 m2 per year in the sparsentan group and –3.9 and –3.8 mL/min/1.73 m2 per year in the irbesartan group. Between-group differences were, respectively, 1.0 mL/min/1.73 m2 per year (95% CI: –0.03, 1.94; p=0.058) and 1.1 mL/min/1.73 m2 per year (95% CI: 0.1, 2.1; p=0.037).18
These findings, postulated Latus, represented very low eGFR decreases in patients with IgAN. However, although the between-group difference in chronic slope was only 1.1 mL/min/1.73 m2 per year, Latus discussed how this can be extrapolated to delayed time to kidney failure in the long term. Utilising data from the PROTECT trial alongside findings from studies of other medications for IgAN, Figure 1 illustrates theoretical delayed times to kidney failure. In this scenario, treatment was initiated in a patient who had an eGFR of 57 mL/min per 1.73 m², which was similar to the mean baseline eGFR of patients enrolled into PROTECT. Projecting the slope of eGFR decrease found in the PROTECT study, it was estimated that the patient would reach kidney failure (eGFR <15 mL/min per 1.73 m²) in 15.6 years if taking sparsentan and 11.1 years if taking irbesartan, representing a difference of 4.5 years, and in 7.9 years if on RASi standard of care, representing a difference between this and sparsentan of 7.7 years.18
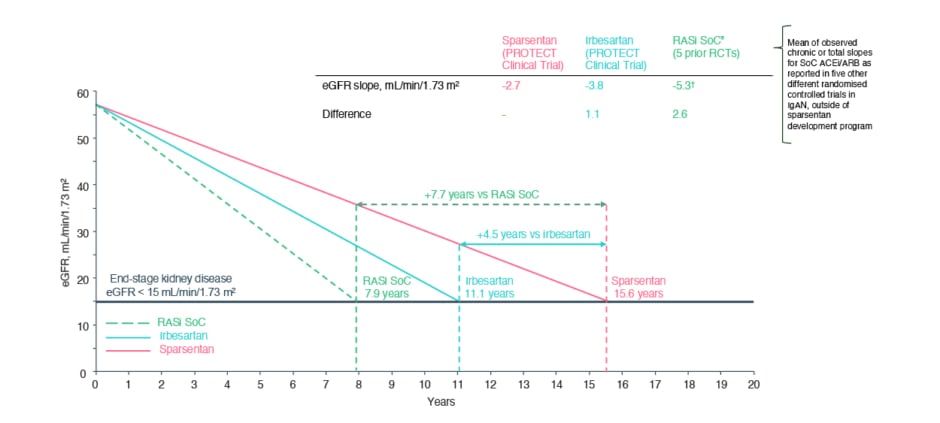
Figure 1: Theoretical/projected delayed time to kidney failure based on linear extrapolation of estimated glomerular filtration rate slopes.18-23
Based on linear extrapolation of the eGFR slopes calculated in the PROTECT study and five randomised controlled trials. These data are extrapolated and act under the assumption that eGFR decline is constant and decreases in a linear fashion in the course of the disease, which is not necessarily accurate for real-world, long-term effects. Outcomes may vary on an individual basis. Outcomes presented are not efficacy outcomes and should be validated in the future with long-term data as they become available.
*ACEi and/or ARB.
†Mean of observed chronic or total slopes for SoC, as reported in five IgAN randomised controlled trials.
ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; eGFR: estimated glomerular filtration rate; IgAN: immunoglobulin A nephropathy; RASi: renin-angiotensin system inhibitor; SoC: standard of care.
While, in the PROTECT trial, treatment-emergent adverse events (TEAE) more often present in the sparsentan group included hypotension (13%) and dizziness (15%), compared with, respectively, 4% and 6% in the irbesartan group, Latus reported that sparsentan overall had “a very good safety profile”.18
Sparsentan Use Beyond Clinical Trials
To understand the use of sparsentan in a real-world setting, Latus shared an analysis of real-world data from Stuttgart, Germany, on the use of sparsentan in patients with IgAN with an eGFR >30 mL/min per 1.73 m² and UPCR >0.75 g/g. Results presented included 23 patients with a median age of 38 years, 57% of which were male, with a median time from initial kidney biopsy to sparsentan initiation of 34 months. These patients had a median baseline eGFR of 42 mL/min per 1.73 m², UPCR of 1.5 g/g, and blood pressure value of 130/80 mmHg, with 65% exhibiting arterial hypertension. At baseline, all patients were being treated with a RASi, with 74% on a maximal indicated dose. All had been treated stably with an SGLT2i in the past, and 57% had a history of glucocorticoid therapy (17% ongoing with MRB at screening).24
Efficacy results showed a significant decrease in UPCR from baseline at Week 2 (n=20), Week 10 (n=21), Week 14 (n=18), and Week 22 (n=8). Proteinuria reduction was >50% in 87% of participants, >30% in 9%, and ≤30% in 4% of participants. Also shown was that 35% achieved complete proteinuria remission and 52% showed partial remission (Figure 2[.hl]).24
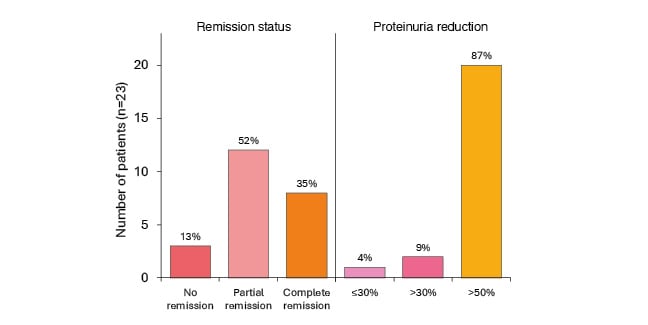
Figure 2: Remission status and proportion of patients reaching complete/partial remission of proteinuria.24
Definition of proteinuria at least once at any time over the course of the treatment period: complete remission is 0.3 g/g, and partial remission is 1.0 g/g if baseline between 0.75−1.0 g/g, then <0.75 g/g.
Sparsentan was generally well tolerated with no evidence of serious adverse events. Hypotension occurred in 8.7% of patients (two cases). One of these patients temporarily ceased treatment for 4 weeks and experienced no hypotension when the treatment was restarted. There were also reports of dizziness, headache, mild hyperkalaemia, gout, and pruritus, each affecting one patient. No patient had to permanently discontinue sparsentan treatment due to a treatment-related adverse event.24 While preliminary, these results show the utility of sparsentan for proteinuria reduction in a real-world setting.
Sodium-Glucose Cotransporter-2 Inhibitors
Latus also discussed the use of SGLT2is, which are now recommended in the 2024 draft KDIGO guidelines for “patients at risk of progressive kidney function loss”.8 A meta-analysis of trials with an SGLT2i showed a relative risk of kidney disease progression of 0.49 (95% CI: 0.32, 0.74). Of note though, Latus said: “Keep in mind that the study population investigated in these trials were an older population with less eGFR compared to the IgAN trials, and that the RAS inhibition was not optimised.”15
To understand the effects of combination therapy, one ongoing trial is investigating 12 weeks of treatment with an SGLT2i added to sparsentan (after it has been taken for ≥8 weeks), compared with sparsentan alone. This includes around 60 participants from the PROTECT open-label extension period. Another study, SPARTACUS, includes participants on stable SGLT2i and RASi treatment where the RASi was discontinued and sparsentan is introduced for 24 weeks. These studies will evaluate safety and efficacy of this combination.25
Modified-Release Budesonide
Another updated recommendation in the draft 2024 KDIGO guidelines for patients at risk of progressive kidney function loss is a 9-month course of a modified-release formulation of the glucocorticoid budesonide (MRB).8,16 Investigation of this formulation was carried out in the Phase III, randomised, placebo-controlled NefIgArd trial. This included 364 adults with persistent proteinuria ≥1.0 g/day despite optimised treatment. MRB was administered at a dose of 16 mg/day for 9 months, compared to a placebo group also on optimised treatment. Findings included a significant percentage decrease in UPCR from baseline, which was also significantly different from the placebo group. Continued decreases were shown up to 12 months even though MRB was stopped at 9 months. While there was a subsequent increase in proteinuria 2 years from baseline, levels were similar to those shown at 9 months.23
An increase was shown in eGFR at Month 3 in the MRB group, with a decrease in the placebo group; however, by Month 9, while decreases remained in the placebo group, eGFR was at baseline in the MRB group. At follow-up, 2 years from study start, although eGFR declined in both groups, following a similar slope, the change from baseline in the MRB group was lower than that of the placebo group.23
In this study, discontinuations in the 9-month treatment period due to TEAEs occurred in 17 (9%) patients in the MRB group and three (2%) in the placebo group. The incidences of infections were similar between groups, at rates of 35% in the MRB group and 31% in the placebo group. Latus reported that TEAEs were similar to what he would expect to be associated with glucocorticoid treatment, such as peripheral oedema (17%), hypertension (12%), muscle spasms (12%), and acne (11%). There were no incidences of new-onset diabetes and no treatment-related fatalities.23
Clinical Trials Aimed at IgAN Pathogenesis
Latus highlighted how KDIGO guidelines recommend that patients with proteinuria >1.0 g/day despite at least 3 months’ supportive care should be considered for enrolment in a clinical trial.7,8 One such trial, the international VISIONARY Phase II trial, is investigating the drug sibeprenlimab plus standard care over 12 months, compared to placebo plus standard care.26 This humanised IgG2 monoclonal antibody binds to and neutralises the activity of the B cell regulator APRIL.6 By blocking APRIL, it is hoped to reduce levels of galactose-deficient IgA1 along with associated immune complexes. Patients aged ≥18 years with biopsy-confirmed IgAN (n=155) were randomly assigned on an equal basis to receive placebo or 2, 4, or 8 mg/kg sibeprenlimab per day. They were stratified at screening according to 24-hour UPCR (≤2.0 versus >2.0 g protein/g creatinine). Results after 12 months showed a dose-dependent, “significant linear treatment effect in change from baseline in 24-hour UPCR (p<0.001)”. In line with the actions of this drug at the Hit 2 level,6 a dose-dependent decrease in serum gd-IgA1 levels, as well as IgG, IgM, and APRIL, over time was shown in all active treatment groups. There was no evidence of a treatment-related adverse toxic effect or of clinically meaningful immunosuppression or infections over the study period.27
The vasoactive peptide endothelin-1 (ET-1) is involved in chronic kidney disease pathogenesis via its action at the endothelin type A receptor (ETA) receptor.28 In the international, randomised, ALIGN Phase III study (n=340), investigation was carried out of the ETA receptor antagonist atrasentan versus placebo in patients with biopsy-proven IgAN and baseline total proteinuria >1.0 g/day despite optimised RASi treatment.29,30 Interim analysis from baseline to Week 36 (n=270) showed that atrasentan administration (0.75 mg/day) led to a 38.1% reduction from baseline in UPCR (median baseline: 1.4 g/g) compared with a 3.1% reduction with placebo (median baseline: 1.4 g/g). Relative reduction in mean percentage change for atrasentan compared with placebo was –36.1% (95% CI: –44.6, –26.4; p<0.001). Reductions in proteinuria with atrasentan were shown at the first assessment at 6 weeks. Atrasentan was generally well-tolerated with a favourable safety profile; TEAEs in the safety set were balanced between the treatment arms.31
The ongoing APPLAUSE-IgAN multicentre, randomised, Phase III trial is investigating iptacopan, a proximal complement inhibitor that binds factor B and inhibits the alternative complement pathway.32,33 The study includes 470 adults with primary IgAN administered 200 mg iptacopan twice daily or a placebo. Interim results at 9 months (n=250) showed reductions from baseline in 24-hour UPCR of 43.8% in the iptacopan group (from median 1.8 g/g at baseline) compared with 9.0% in the placebo group (from median 1.9 g/g at baseline). Relative percentage reduction was 38.3% (95% CI: 26.0, 48.6; p<0.0001). Iptacopan was well-tolerated and had a favourable safety profile, with the rate of TEAEs leading to discontinuation similar between the treatment arms.34
These studies, Latus said, show promising results and point to future questions regarding tailoring specific treatments to particular patients, taking into account autoimmunity and chronic kidney disease progression.
Finally, Latus discussed the importance of looking at results for the comparator group in clinical trials with regard to between-group differences (Table 1). “When we talk about treatment effects,” he said, “we always have to keep in mind how the other group was doing.” Understanding this can help guide more individualised treatment choices.
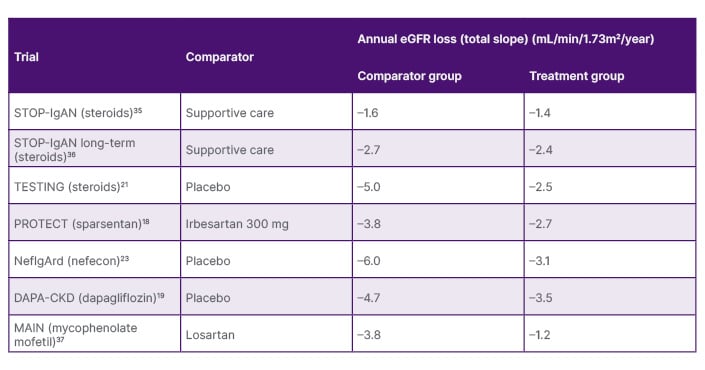
Table 1: Treatment effects in clinical trials.
Note, the clinical trials presented on this slide have used different study designs. Direct comparisons should not be made, and data should be interpreted with caution.
eGFR: estimated glomerular filtration rate.
Conclusion
Updated 2024 draft KDIGO guidelines recognise the importance of proteinuria remission and expand recommended treatment choices for patients with IgAN at risk of progressive kidney failure to include sparsentan, an SGLT2i, and MRB.8 This will help provide more tailored therapy to patients on a more individualised basis. With a number of other treatments in trial, a lot more therapies will come in the future, concluded Latus.







