Meeting Summary
IgA nephropathy (IgAN) can impact life expectancy in those affected, thus efficacious treatment is key. Endothelin 1 (ET-1) and angiotensin II (Ang II) are instrumental in the development of IgAN-associated renal damage. Use of sparsentan, a dual ET Type A receptor (ETAR) and Ang II subtype 1 receptor (AT1R) antagonist (DEARA), can lead to reductions in proteinuria and thereby help to slow kidney function decline in patients with IgAN. Sparsentan is included in the 2024 draft Kidney Disease Improving Global Outcome (KDIGO) guidelines for patients with IgAN at risk of progressive kidney function loss. Additionally, other KDIGO guidelines recommend a sodium-glucose cotransporter-2 inhibitor (SGLT2i) for all adults at risk of chronic kidney disease (CKD) progression as they are associated with reductions in both kidney and cardiac morbidity and mortality risks. If needed, there is the potential to combine these drugs. Studies regarding such use were presented in three posters at the American Society of Nephrology’s (ASN) Kidney Week 2024. The first included updated interim findings from the open label extension (OLE) study of the Phase III PROTECT trial of sparsentan in a subset of participants where an SGLT2i had been added at the investigators’ discretion. Here, SGLT2i addition led to further proteinuria reduction. The second poster detailed a prespecified interim analysis of the SPARTACUS trial wherein participants with IgAN receiving an SGLT2i and angiotensin-converting enzyme inhibitor (ACEi)/angiotensin receptor blocker (ARB) treatment were switched to sparsentan plus an SGLT2i. Such a regimen led to proteinuria reduction from baseline. In the final poster, including four patients with IgAN in tertiary care, the concomitant use of sparsentan with an SGLT2i led to proteinuria reduction in the real-world setting regardless of proteinuria levels or kidney function at sparsentan initiation. Taken together, these studies suggest the utility of concomitant use of sparsentan with an SGLT2i in patients at risk of kidney failure progression.
Introduction
IgAN is an immune-complex mediated glomerular disease that typically first occurs in young- to mid-adulthood1,2 and is more common in males.3 Though rare, with an incidence rate of up to 5.7 per 100,000 per year, IgAN is a leading cause of primary glomerulonephritis.2,4 Disease progression in IgAN may be slow in some, but rapid in others.5,6 Symptoms can also vary, ranging from asymptomatic microscopic haematuria to rapid decline in renal function.7,8 Sustained proteinuria strongly predicts kidney function decline, with each incremental g/day >1 g associated with a 10- to 25-fold more rapid rate of decline.9 According to one study, compared with sex and aged-matched controls, IgAN confers a 6-year reduction in life expectancy and a 1.53-fold increased risk in all-cause mortality.8 Another study of adults with IgAN showed an estimated 20-year survival rate of 0.28 (95% CI: 0.25, 0.31).10 As such, IgAN is an important disease to recognise and control.
IgAN can develop due to an inherited abnormality and/or following a mucosal infection.11,12 The result is increased levels of galactose deficient IgA1 (Gd-IgA1) and subsequent production of Gd-IgA1 antibodies, followed by deposition of Gd-IgA1-containing immune complexes in mesangial cells. Resulting cell activation and proliferation is stimulated by, and in turn stimulates, several mediators including ET-1 and Ang II. ET-1 has a role in vascular tone and glomerular arteriolar regulation, as well as in fluid, sodium, and renin-angiotensin-aldosterone system homeostasis.13 Rapid IgAN progression is associated with increased ET-1 renal expression14 and sustained activation of the ETAR is associated with fibrosis, tubulointerstitial inflammation, and proteinuria in IgAN.15 Ang II can enhance vasoconstriction caused by ET-1 and stimulate renal release of this molecule.16,17 Via the AT1R, Ang II is involved in proteinuria development, as well as tubulointerstitial fibrosis, renal inflammation, and vascular dysfunction in CKDs.17
According to draft 2024 KDIGO guidelines (with note that final guidance may change based on feedback), patients with IgAN at risk of progressive loss of kidney function are those with proteinuria ≥0.5 g/d (or equivalent) with or without treatment. Treatment goals for such patients include reduction ‘in the rate of loss of kidney function to <1 mL/min per year for the rest of the patient’s life.’18 This should be guided by urine protein excretion, which, the guidelines stipulate, ‘should be maintained at <0.5 g/d (or equivalent) and preferably <0.3 g/d (or equivalent).’ 18
Along with lifestyle advice, cardiovascular risk assessment, and blood pressure control, the draft guidelines state that management of IgAN-induced nephron loss should include ‘measures to reduce glomerular hyperfiltration and the impact of proteinuria on the tubulointerstitium.’ Treatment to achieve this goal includes the use of renin-angiotensin system blockade or a DEARA with or without an SGLT2i.18
Sparsentan is a once-daily, oral, novel, non-immunosuppressive, single-molecule, highly selective DEARA that directly targets glomerular injury in the kidney.17,19 It has been shown to have protective, preservative, antifibrotic, and anti-inflammatory effects on a variety of kidney structures and mechanisms.20 The Phase III PROTECT trial showed that sparsentan administration led to sustained proteinuria reduction and kidney function preservation in patients with IgAN.21 Sparsentan is fully approved in the USA to ‘slow kidney function decline in adults with primary IgAN who are at risk for disease progression,’22 and has received conditional marketing authorisation in the EU (April 2024) for adults with IgAN with a urine protein-to-creatinine ratio (UPCR) ≥0.75 g/g.23
SGLT2is work to enhance urinary glucose excretion by reducing glucose reabsorption in the kidney proximal convoluted tubule.24 While developed for treatment of Type 2 diabetes, SGLT2is can also reduce albuminuria and slow the rate of estimated glomerular filtration rate (eGFR) decline,25,26 including in non-diabetic patients with CKD-associated proteinuria.27 Subgroup analysis of the ‘Dapagliflozin and Prevention of Adverse Outcomes in CKD’ (DAPA-CKD)28 and the ‘EMPAgliflozin once daily to assess cardio-renal outcomes in patients with chronic KIDNEY disease’ (EMPA-KIDNEY) trials29 showed that SGLT2i administration may be associated with reduced risk of progression to kidney failure in patients with IgAN,28,29 which was 0.49 (95% CI: 0.32, 0.74) in a meta-analysis of these trials.30
With these findings in mind, the combination of sparsentan with an SGLT2i is postulated to provide therapeutic benefits, including kidney protection, for patients with IgAN at high risk of disease progression. At the ASN’s Kidney Week 2024, three posters were presented highlighting the efficacy and safety of the combination of sparsentan and an SGLT2i in clinical trial and real-world settings.
Updated Results from the Ongoing Protect Study Open-label Extension: Adding an SGLT2i to Sparsentan According to Need
In the PROTECT trial double-blind period, patients were randomised to either sparsentan 400 mg/day or irbesartan 300 mg/day for 110 weeks.21 Participants who completed this period could enter the PROTECT OLE. Following a 4-week study drug withdrawal period (during which participants received ACEi/ARB treatment) all participants received sparsentan to a target dose of 400 mg/day, between Weeks 114 and 270. At the investigators’ discretion, a concomitant SGLT2i could be initiated at any time during the OLE. Of note, a separate substudy of the PROTECT OLE is being carried out where participants are randomised to receive an SGLT2i, participants in this substudy were excluded from this analysis.31,32
Early clinical data (2023) from 21 participants in the PROTECT OLE showed that adding an SGLT2i to ongoing sparsentan treatment demonstrated a benefit regarding proteinuria reduction and was generally well-tolerated.33 Presented in this current poster were updated data from 61 patients who received at least one dose of a concomitant SGLT2i. The baseline was defined as the OLE visit closest to SGLT2i initiation.32
In this cohort, 70% were male, 70% were White, 21% were Asian, and 3% were Black/African American. They had a mean (standard deviation [SD]) age of 46.2 (11.7) years and median (interquartile range [IQR]) time from start of OLE treatment to SGLT2i initiation of 261.0 (148.0−411.0) days. Of the 61 participants, at SGLT2i initiation, 80% had a history of hypertension and 66% were taking antihypertensive medications, including calcium channel blockers (43%), diuretics (34%), beta-blockers (25%), and alpha-blockers (11%).32
At baseline, median (IQR) UPCR was 1.3 (0.8−2.3) g/g. As shown in Figure 1A, the combination of sparsentan and an SGLT2i led to a reduction in proteinuria at Week 12 that remained at Week 48 (evidenced by percentage change from baseline in UPCR geometric mean).32 Body weight (Figure 1B) and blood pressure (Figure 1C) were relatively stable over the 48 weeks, with slight reductions in eGFR (Figure 1D) over this period.32
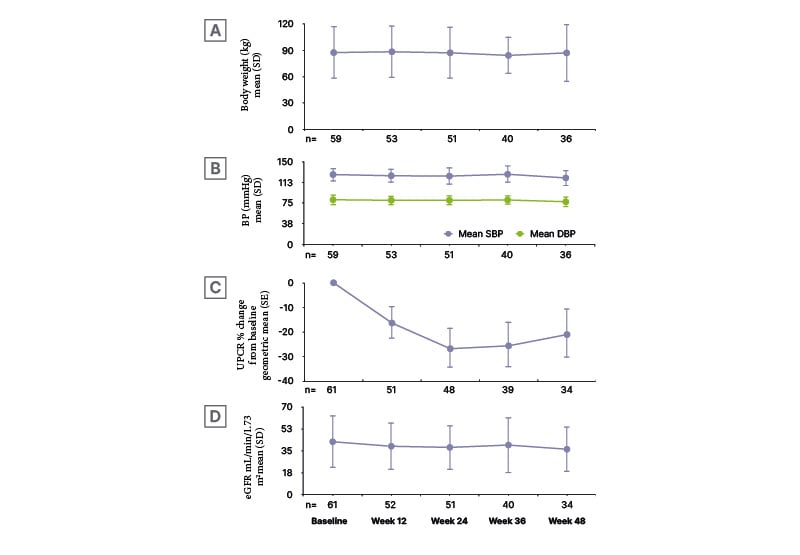
Figure 1: A) Percentage urine protein-to-creatinine ratio change from baseline, B) body weight, C) blood pressure, and D) estimated glomerular filtration rate over 48 weeks.32
Baseline: OLE visit closest to start of SGLT2i medication.
DBP: diastolic blood pressure; eGFR: estimated glomerular filtration rate; SBP: systolic blood pressure; SD: standard deviation; SE: standard error; UPCR: urine protein-to-creatinine ratio.
The addition of an SGLT2i to sparsentan was generally well-tolerated. Forty-six (75%) participants had a treatment-emergent adverse event (TEAE). TEAEs in ≥5% of patients included hyperkalaemia (11%), COVID-19 (10%), hypertension (7%), and hypotension (7%), as well as CKD, cough, decreased GFR, peripheral oedema, and upper respiratory tract infection (all 5%). Seven participants (11%) had a serious TEAE, including 3% (two participants) with hyperkalaemia, and 2% (one participant each) reporting acute kidney injury, CKD, COVID-19, headache, small intestinal obstruction, spontaneous abortion, and umbilical hernia. No cases of Hy’s law were reported (alanine aminotransaminase or aspartate aminotransaminase >3x upper limit of normal plus total bilirubin >2x upper limit of normal).32
The authors concluded that data are consistent with an additive benefit on proteinuria reduction with combination therapy of stable sparsentan plus an SGLT2i.32
Prespecified Interim Analysis of the Phase II SPARTACUS Trial: Combination of Sparsentan and an SGLT2I
The efficacy and safety of the combination of sparsentan and an SGLT2i is also being explored in the ongoing, exploratory, open-label, single-arm, multicentre, Phase II SPARTACUS trial. Eligibility criteria include being aged ≥18 years with biopsy-proven IgAN, a urine albumin-to-creatinine ratio (UACR) of ≥0.3 g/g, and an eGFR of ≥25 mL/min/1.73 m2 despite stable use of an SGLT2i and maximum labelled dose of ACEi/ARB therapy for ≥12 weeks. At baseline, ACEi/ARB use was stopped, and then sparsentan (400 mg/day) was added to the SGLT2i for 24 weeks.34
The primary efficacy endpoint in SPARTACUS is change from baseline in UACR at Week 24. Secondary efficacy endpoints are achievement of UACR of <0.2 g/g, and 30% and 50% reduction in UACR at Week 24, as well as change from baseline in UACR, UPCR, eGFR, and blood pressure at each visit. Changes are analysed with a mixed model repeated measures approach. Safety is also being assessed.34,35
In the poster presented here, data from 20 patients in a prespecified interim analysis at Week 24 were described. Mean (SD) age was 52.9 (15.0) years, weight was 90.9 (22.8) kg, and BMI was 31.4 (5.6) kg/m2. Patients were predominantly male (65%) and were White (55%) or Asian (45%). Median (IQR) UACR at baseline was 0.84 (0.52−1.26) g/g, with UPCR being 1.45 (1.06−2.40) g/g. Mean (SD) eGFR was 54.8 (20.6) mL/min/1.73 m2, and systolic/diastolic blood pressure was 129.8 (12.5)/78.8 (10.9) mmHg. Haematuria was present in 50% of participants and glycosuria in 90%.35
As can be seen in Figure 2A, sparsentan plus an SGLT2i led to rapid (at Week 4) and sustained reduction in UACR. The percentage UACR change at Week 24 was −39.5%. At this time point, switching from ACEi/ARB therapy to sparsentan on a background of an SGLT2i allowed patients to reach target response endpoints, as evidenced by 16% of participants with a UACR <0.2 g/g, along with nearly two-thirds having a ≥30% reduction in UACR from baseline and one third having a ≥50% reduction from baseline. EGFR levels were relatively stable (Figure 2B) and there were slight blood pressure decreases (Figure 2C).35
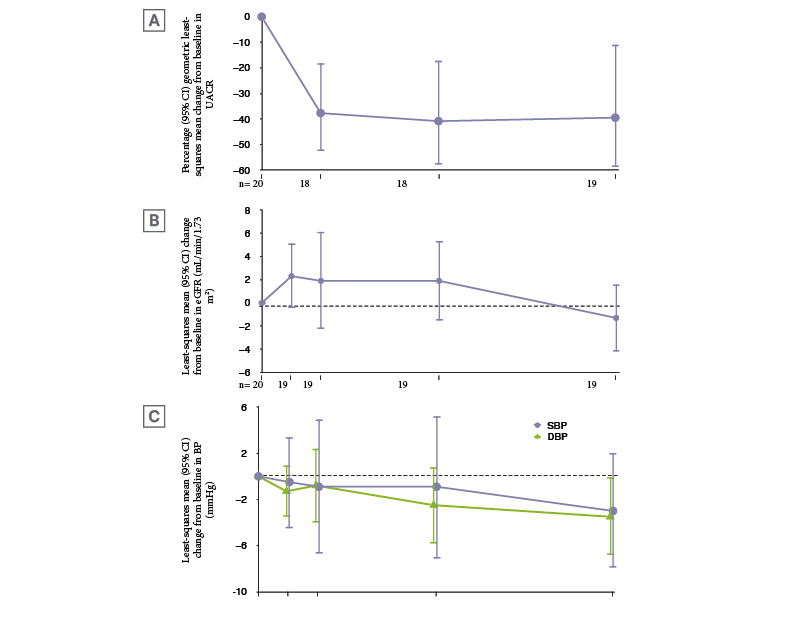
Figure 2: Changes in A) urine albumin-to-creatinine ratio, B) estimated glomerular filtration rate, and C) blood pressure at each visit.35
CI: confidence interval; DPB: diastolic blood pressure; eGFR: estimated glomerular filtration rate; SPB: systolic blood pressure; UACR: urine albumin-to-creatinine ratio; Wk: week.
Sparsentan was generally well-tolerated with no unexpected safety signals. Twelve patients (60%) experienced a TEAE, which was deemed sparsentan-related in five participants (25%) and SGLT2i-related in one (5%). TEAEs reported in at least two participants included dizziness, headache, hypertension, hypotension, oedema, peripheral oedema, and osteoarthritis (all occurring in 10% of patients). There was one severe TEAE of gout (5%), and three serious AEs of cerebrovascular accident in one patient, and, in the same patient, osteoarthritis and acute kidney injury (which was mild, unrelated to sparsentan or SGLT2i treatment, and resolved after treatment interruption). One TEAE of vertigo led to treatment discontinuation. No participant displayed any abnormal liver function test results.35
Similar to the PROTECT conclusion, the authors here surmised that these findings following the addition of an SGLT2i to stable sparsentan ‘are consistent with an additive benefit on proteinuria reduction’ such that the combination ‘represents an effective treatment with a good safety profile for patients with IgAN.’35
Real-World Evidence: A Case Series of Sparsentan in Combination with an SGLT2I
While the above studies show the efficacy and safety of the combination of sparsentan and an SGLT2i in the trial setting, real-world evidence is still limited. The final poster reported data for one male and three female patients who attended a tertiary care centre and were selected by their healthcare provider for inclusion in this case series. All patients were White with an age range of ~25−65 years and biopsy-proven IgAN diagnosed from between 4 months to 11 years before baseline. Prior to receiving the sparsentan/SGLT2i combination, three patients received steroid/immunosuppressive treatment alone or in combination with a renin-angiotensin system inhibitor. One patient received losartan alone. These treatments were discontinued prior to sparsentan/SGLT2i initiation.36
Figure 3 shows data from when the patients were initiated on sparsentan to the time of analysis. All patients received sparsentan initially at a dose of 200 mg/day, increasing to 400 mg/day after 2 weeks (or closest follow-up visit), as per label instructions. Patients received the SGLT2i dapagliflozin for ~3−5 months. As shown in Figure 3, at the time of analysis, all patients had been receiving both drugs for ~2 months. While three of the four patients were either already receiving dapagliflozin or were initiated on it concurrently with sparsentan, for one, dapagliflozin was initiated ~1 month after sparsentan initiation.36
Figure 3A shows UPCR from baseline for three of the patients, with Figure 3B showing UACR for the fourth. Decreases in these measures of proteinuria from baseline (38−82% change in UPCR and a 95% change in UACR) occurred in all patients regardless of eGFR at initiation (Figure 3C).36
As previously discussed, in the 2024 draft KIDGO guidelines, urine protein excretion should be maintained at preferably <0.3 g/d and at least <0.5 g/d.18 Here, one patient (who received 1 month of sparsentan treatment alone, then the combination for 3 months) achieved UACR 0.16 g/g (Figure 3B), and two patients achieved UPCR of 0.4 g/g, one after 3 months’ combination therapy and one where dapagliflozin was added for 2 months following 8 months’ sparsentan treatment (Figure 3A).36
At sparsentan initiation, all patients had haematuria, which resolved in one patient at follow-up. The sparsentan plus dapagliflozin combination was generally well-tolerated with relatively stable blood pressure.36
The authors concluded that this case series ’supports the safety and effectiveness of sparsentan in combination with the SGLT2i dapagliflozin in patients with IgAN.’ Proteinuria improvements were observed regardless of eGFR or UPCR/UACR at sparsentan initiation. These cases also highlight greater proteinuria improvements achieved with the sparsentan and SGLT2i combination than with previous treatments.36
Conclusion
The findings shown in these three posters highlight how sparsentan in combination with an SGLT2i represents an effective treatment regimen with a well-tolerated safety profile for patients with IgAN at risk of progressive kidney function decline.
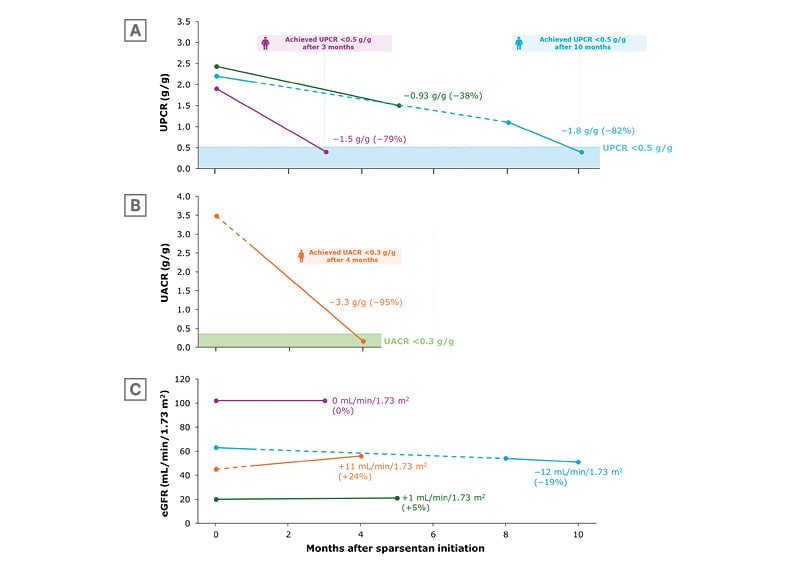
Figure 3: Change in A) urine protein-to-creatinine ratio, B) urine albumin-to-creatinine ratio, and C) estimated glomerular filtration rate following initiation of sparsentan.36
Solid lines indicate sparsentan + SGLT2i combination treatment; dashed line indicates sparsentan administered alone. Values reflect mean (%) change from sparsentan initiation (Month 0) to last follow-up.
eGFR: estimated glomerular filtration rate; UACR: urine albumin-to-creatinine ratio; UPCR: urine protein-to-creatinine ratio.







