Summary
Wilson’s disease (WD) occurs due to excess copper leading to hepatic or neurologic symptoms, or a combination of both. Treatment for symptomatic WD typically includes initial therapy with a chelator, such as D-penicillamine or trientine, with zinc salts recommended in some guidance documents for neurologic WD, although this is not universally accepted. For presymptomatic patients, guidelines suggest either chelators or zinc as initial therapy. Zinc therapy has been used for over 60 years, with effectiveness demonstrated in a number of studies or retrospective case reviews. However, there are few head-to-head controlled trials of zinc compared to chelators. Accordingly, more such trials are needed to understand the use of zinc for WD. The main adverse event associated with zinc therapy is gastrointestinal issues, which can be a limiting factor in the usefulness of zinc. Copper deficiency may occur in a minority of patients taking zinc, with early warning signs including leukopenia and pancytopenia. Further studies are needed to understand the mechanism of action of zinc for WD, and long-term studies are also needed to understand the adverse event profile. Zinc can be an effective and safe choice for WD treatment; however, further studies are needed to fully understand the mechanism of action and use.INTRODUCTION
Zinc salts are one of three approved treatments for the autosomal recessive condition Wilson’s disease (WD). Their use was first described in 1961, and further developed from the 1970s.1,2 However, while in some countries zinc is increasingly used to treat WD, in others, use is less evident. Along with the discussion about zinc’s current use for WD, the authors explore whether the reluctance to recommend or use this therapy may in part be due to a paucity of scientific knowledge, as well as inexperience in using zinc compared to chelators. Knowledge gaps in the understanding of the use of zinc for patients with WD, and where there may be study opportunities, will be assessed.
WD onset is typically before age 40 years,3 with prevalence varying, depending on ethnicity, from 1:17,000−50,000 people.4,5 In paediatric patients, WD more typically presents as liver disease (hepatic Wilson’s disease [hWD]), which can range from asymptomatic cytolysis to acute liver failure, and includes elevated liver enzymes, splenomegaly, fatty liver, acute hepatitis, and cirrhosis.6,7 In adolescents and adults, initial presentation is commonly neurologic/psychiatric (neurologic Wilson’s disease [nWD]), with symptoms including dysarthria, seizures, sleep disorders, dystonia, ataxia, hypokinesia, and tremor; and in some, depression, anxiety, personality changes, and psychosis.7-9 Around 95% with nWD and 50% with hWD show corneal copper Kayser–Fleischer rings.10
In WD,11 copper accumulation in the liver, brain, and other organs7,12,13 occurs due to functional deficits of copper ATPase, attributable to variants in ATP7B.14,15 This enzyme is involved in the binding of excess copper in hepatocytes by intracellular metallothioneins. Copper is then excreted in bile or incorporated into plasma ceruloplasmin. Metallothioneins can also bind dietary copper, which is retained within intestinal cells until they are sloughed off and excreted.7
WD can be fatal, so early diagnosis and treatment are vital.16 Symptomatic WD is typically first treated with chelators to promote copper excretion, then in-range copper levels are maintained with a chelator or zinc.7,12,17-21 The chelator D-penicillamine (DPA) mobilises and binds copper from tissues; trientine promotes copper excretion and decreases intestinal copper absorption. Ammonium tetrathiomolybdate may also be used, but clinical experience and drug availability is limited.7,9,12,17-21 Adverse events (AE) associated with DPA include neurological worsening, cutaneous eruptions, lymphadenopathy, neutropenia, thrombocytopenia, and proteinuria; as well as later, rarer, nephrotoxicity, lupus-like syndrome, Goodpasture syndrome, and elastosis perforans serpiginosa9,17-19,21,22 AEs with trientine include early- or later-onset hypersensitivity reactions as well as rare lupus-like reactions, pancolitis, and sideroblastic anaemia.9,17-19,21
ZINC MECHANISM OF ACTION
The mechanism of action of zinc in WD is not fully elucidated. It is known that on administration, intestinal zinc can greatly increase intracellular metallothionein expression. As metallothioneins preferentially bind copper over zinc, this action can prevent intestinal and hepatic copper absorption/reabsorption.2,7,23-25 Additionally, zinc may directly reduce oxidant liver injury, which can occur due to excess copper.26 However, while the time lag from first zinc administration, typically around 3 weeks, is hypothesised to be due to the time needed to increase metallothionein levels and restore zinc balance,1 it is not clear why this lag varies between patients,1 and why in some patients zinc has no measurable effect.27
Animal models show that high copper levels may directly disrupt zinc levels and associated mechanisms, such as zinc’s role in lipid metabolism.28 Zinc itself may also affect other metals, such as iron, with which it shares a metal transporter into duodenal enterocytes.29 As such, zinc supplementation may both decrease copper levels, and restore zinc balance and zinc-dependent functions.28 Further work is needed to fully understand these mechanisms.
USE OF ZINC THERAPY
Zinc salts include acetate (ZA), sulphate (ZS), gluconate (ZG), or picolinate.17 The recommended dose is 50 mg 3x /day in adults, or 25 mg 2−3x /day in children, ≥1 hour before and ≥2 hours after eating for best absorption. This should maintain in-range copper levels while limiting effects if a dose is missed.30-32 While there is little perceived difference in copper control between zinc salts, ZS may be associated with more gastrointestinal (GI) AEs, although there is wide inter-individual variability that merits investigation.19,27,33,34
Only ZA is approved by the U.S. Food and Drug Administration (FDA) for WD maintenance therapy,32,35 and by the European Medicines Agency (EMA) for all patients.36,37 Studies are needed to directly compare the efficacy and safety of all zinc salts, for them to be regulated similarly to ZA, and thus available for prescribing. These are important, as in a retrospective review of 31 patients who were recommended zinc, only 45% were taking ZA, with 42% taking non-prescription ZG, and the remainder taking ZS or picolinate. Additionally, it was found that 51% purchased their zinc salt without a prescription.38 These findings are of concern as problems may occur with regard to content and quality when buying unregulated zinc salts intended as dietary supplements, and not otherwise tested.38 Of concern too, non-prescription medication use is typically not formally recorded by a healthcare professional, leaving ‘ghost patients’ with WD who are taking zinc without any record of the type, dose, effects, or AEs. Studies are needed to understand how patients access zinc, what salts they use, and why they access it without a prescription, which may be for financial, availability, or AE issues.
Studies of Zinc for Wilson’s Disease
EMA/FDA approvals for ZA31,32,35 were based on uncontrolled clinical trials and case studies ( Table 1). These, along with post-authorisation reviews, show that zinc therapy leads to adequate copper control and WD symptom stabilisation or improvement in both pre-symptomatic and symptomatic patients, and those initiated on chelation therapy and then switched later. Disease progression, in a minority of patients, was typically due to non-adherence.31,35,38-42
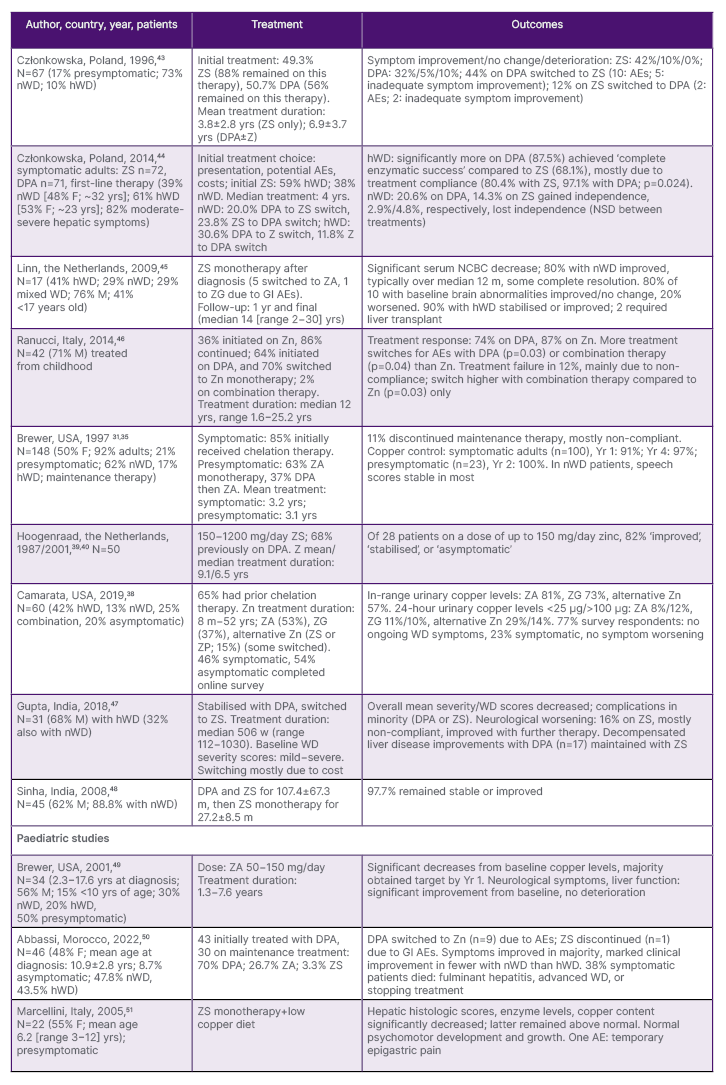
Table 1: Selected studies on the use of zinc therapy for Wilson’s disease.
AE: adverse events; DPA: D-penicillamine; F: female; GI: gastrointestinal; hWD: hepatic Wilson’s disease; M: male; m: months; N: number; NCBC: non-ceruloplasmin-bound copper; NSD: no significant difference; nWD: neurologic Wilson’s disease; USA: United States of America; w: weeks; yr: year; yrs: years; Zn: zinc salts; ZA: zinc acetate; ZG: zinc gluconate; ZP, zinc picolinate; ZS: zinc sulphate.
Zinc as Initial Therapy and Monotherapy
The use of zinc for initial therapy has gained attraction, with one study showing it is used in around 50% of Polish National Reference Centre patients.52 While the EMA states that, “in principle, ZA is not recommended for initial therapy of symptomatic patients because of its slow onset of action,”31 it is noted that full symptom control with chelators may also be delayed due to the recommendation for titrated initiation to avoid neurological worsening.20,21 As such, investigation is needed into the time of onset of action comparing zinc to titrated chelators, to ascertain if there are differences that may affect the patient.
Although studies have been carried out with at least some patients initiated on zinc ( Table 1),31,35,38-40,43-46 few provide direct comparison to chelators. In one that did, no significant differences were found between ZS or DPA in percentages achieving ‘therapeutic success’, although a significantly higher percentage of patients with hWD receiving DPA achieved ‘complete enzymatic success’. The authors concluded that “ZS may be considered a reasonable alternative to DPA as first-line therapy in all patients with WD.”44 A 2019 review by National Health Service (NHS) England concluded that “the best available evidence suggests zinc salt monotherapy or penicillamine is effective for treating most people with symptoms of WD.”53 However, in another, the conclusion regarding zinc monotherapy was that, “results for symptomatic neurologic disease are highly satisfactory,” but that “clinical outcome was less satisfactory for hepatic disease.”45
To aid the understanding of zinc as initial therapy, more prospective, randomised studies that investigate zinc versus chelators in newly diagnosed patients, as well as in patients with a wide range of presentations, are needed. Previously published retrospective studies evaluated zinc primarily in stable patients. Further prospective studies could help elucidate if there are differences in WD symptom control between patients initiated on zinc or chelator therapy, or those switching from a chelator to zinc. Longitudinal studies are also needed to understand how well WD symptom control is maintained with zinc.
Switching Treatments and Combination Therapy
Although many patients initiated on zinc or DPA continue the treatment, in others, the treatment is switched.31,43,44,46 In one study, while for nWD switching was at a similar rate between treatments (around 22%), for hWD, more patients initially treated with DPA (30.6%) were switched to zinc than vice versa (11.8%), especially in the first 6 months. Therapy switch because of AEs occurred in 15.3% on DPA, mostly due to albuminuria, and 2.6% on ZS (p=0.008). Switch due to lack of improvement was 7.7% for ZS, and 2.8% for DPA (p=0.279).44
DPA may also be switched to zinc due to cost.48 However, two Indian studies concluded that “in resource-constrained settings, sequential DPA followed by zinc therapy may be safe and effective across all degrees of baseline disease severity,”47 and that “withdrawal of DPA from DPA/ZS maintenance therapy was effective, safe, and economic for almost all patients.”48 There are problems though, that when, or how, to switch therapies is not well defined in most guidelines.
Combination zinc/chelator therapy is off-label and rarely studied.18 A pooled analysis54 showed that effectiveness was lower, and AEs higher than with DPA,55 trientine,56 or zinc57 alone, especially for hWD. The mortality rate was highest with a DPA/ZS combination.54 While the Indian Society, American Association for the Study of Liver Diseases (AASLD), and European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) guidelines do suggest combination therapy use ( Table 2), especially for severe disease, drugs should be given at widely spaced time intervals to prevent zinc chelation.19,20,33 However, evidence levels for these recommendations are low, and the efficacy and safety of zinc/chelator combinations need further investigation.
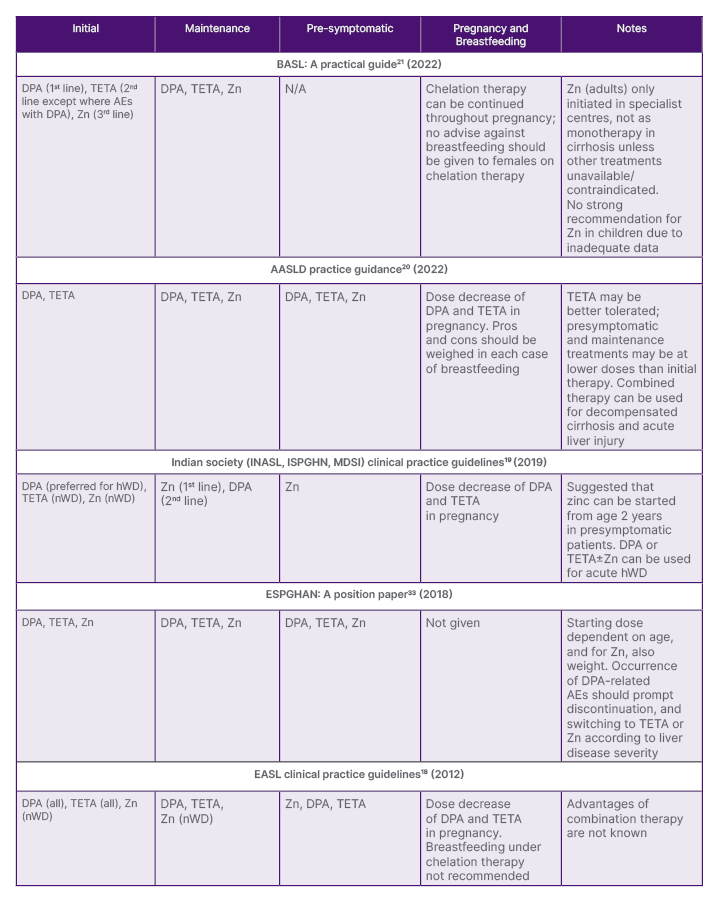
Table 2: Pharmacological treatment guidance for the use of zinc for Wilson’s disease (by date of issue).
AASLD: American Association for the Study of Liver Diseases; AE: adverse event; BASL: British Association for the Study of the Liver; DPA: D-penicillamine; EASL: European Association for the Study of the Liver; ESPGHAN: European Society for Paediatric Gastroenterology, Hepatology and Nutrition; hWD: hepatic Wilson’s disease; INSAL: Indian National Association for Study of the Liver; ISPGHN: Indian Society of Pediatric Gastroenterology, Hepatology, and Nutrition; MDSI: Movement Disorders Society of India; nWD: neurologic Wilson’s disease; TETA: trientine; Zn: zinc salts.
Paediatric Cases
In children, starting doses of all treatments are typically lower, and age- and weight-dependent. In symptomatic patients, zinc can significantly decrease copper levels, and for most, significantly improve neurological symptoms and liver function,49-51 although it was noted in one study that marked improvement was found in fewer with nWD compared to hWD50 (Table 1). Further studies are needed to understand if there are differences in symptom control with zinc therapy between pre-symptomatic and symptomatic children with WD. Longitudinal comparison studies are needed to evaluate drug-related long-term AEs and efficacy, and to understand which therapy is most suitable for lifelong treatment.
Guidance Documents for the Use of Zinc Therapy
The positioning of zinc therapy varies between guidance documents ( Table 2). For example, for initial therapy in symptomatic patients, the AASLD does not recommend zinc,20 the British Association for the Study of the Liver (BASL) places zinc third-line,21 and the European Association for the Study of the Liver (EASL)18 and relevant Indian societies19 guidelines recommend zinc as initial therapy in patients with nWD only. Guidelines also vary with regard to recommendations for pre-symptomatic patients, and for maintenance therapy.18-21,33
As the guidelines are drawn up by experts in the field, the reasons for the differences may be due to more experience, and thus confidence in chelators and low level of available evidence. Indeed, the Indian joint societies guidelines state that “each centre uses a protocol based on their experience and patient compliance.”19 Robust, comparative trials of zinc and chelators that include a homogenous group of patients on which to base recommendations are needed.
SAFETY OF ZINC
Few studies directly compare treatments with regard to safety, but there are indications that while zinc is more likely to be discontinued than DPA due to treatment failure,57 it may have a better safety profile, and be more tolerated than DPA.41-43
Gastrointestinal Symptoms
Overall, the EMA concludes that “gastric irritation is the only AE for which there is good evidence of a causal link to zinc.”31 Across studies, gastric irritation with zinc occurred in 9−65% of patients, with symptoms including a burning sensation in the throat, cough, abdominal pain, nausea, and vomiting.31,38,49,58,59 Where endoscopic evaluation has been warranted, gastric mucosal alterations, including redness, erosions, and for some, ulcers, have been noted with ZA. These were resolved following therapy cessation.59 In one study, ZS administration significantly increased the odds of gastropathy compared to DPA (p=0.01) or no treatment (p=0.02).58 Even though GI AEs are those most associated with zinc,31 there is a lack of distinct recommendations for how to best track all drug-related AEs if these occur, or if mild AEs become serious. Studies are needed to find if AEs are tracked; for example, by using a patient diary or phone application, this could lead to earlier intervention, and better-informed treatment decisions.
As GI symptoms are primarily shown with the first dose of zinc prior to breakfast, patients who experience such may be advised to take zinc between breakfast and lunch (unless this impairs their ability to take zinc 3x /day), or with a small amount of a high protein food.60 Of note, as zinc absorption can be decreased by certain foods,61 co-administration with food is not advised, and further studies are needed to fully ascertain the relationship between zinc absorption and food types in WD. In some studies, switching the zinc salt led to symptom resolution.38 However, this is not recommended in guidelines, and comparison studies of salts are needed to ascertain if GI symptoms, or any AEs occur to a greater extent according to salt, and if an AE does occur, does switching to a different salt lessen such effect.
Copper Deficiency
Development of copper deficiency (CD), typically over months or years, is rare in patients receiving WD therapy (0.9% in one study),62 and predominantly only occurs when zinc is administered alone or with a chelator.63 It occurs potentially due to metallothionein overproduction, and can occur in both symptomatic and presymptomatic patients.62 CD may result in anaemia, neutropenia, or leuconeutropenia, or in more severe cases, lower limb paraesthesia, progressive sensory ataxic gait disorder, and irreversible neurological symptoms.62,64-66 Zinc-induced CD is best predicted by plasma copper levels ≤6 µmol/L combined with plasma zinc levels ≥18 µmol/L (indicating potential overtreatment),67 with warning signs including severely reduced urinary copper excretion, combined with low levels of total serum copper and ceruloplasmin.63 CD usually resolves completely following dose decrease or treatment switching.62 The role of individual metabolism and/or genotype merits investigation.
Further studies regarding zinc’s mechanism of action are needed to understand the relationship with CD, why CD only occurs in some patients, or if occurrence differs between zinc salts. Subclinical CD may not be recognised if proper attention is not paid to the aforementioned warning signs capable of guiding early recognition and prompt reduction or discontinuation of zinc.
Neurological Worsening
Guidance documents point to early neurological deterioration in 8.8−35.3% of patients following WD therapy initiation.18-21,44,57,68 Neurological deterioration is more common in nWD and less common in pre-symptomatic WD.70 While in some studies neurological worsening more often occurred with DPA,44,68,69 in others, occurrence was similar between DPA, zinc, or trientine.57 As treatment switching can lead to gradual neurological improvement,69 it is presumed that this AE is directly associated with therapy; however, there are unknowns that need investigating, including why neurological deterioration occurs only in some patients, the relationship between copper control and neurological worsening, and whether it is greater according to zinc salt.
Liver Enzymes, Pancreatic Enzymes, and Cholesterol
Zinc administration can lead to alanine aminotransferase levels >1−2x the upper level of normal;2,38,70 however, this may not be accompanied by cholestasis31,70 or disease progression.71 While elevated liver enzymes may be due to treatment non-adherence,31 a study showed this even when urinary copper was within target.38 Further studies are needed to investigate the relationship between zinc and hepatic enzymes.
Zinc dose may also influence pancreatic amylase and lipase levels, although this may not lead to pancreatitis symptoms.31,70 One study suggested that dosing normalised for weight or body surface area may limit potentially damaging pancreatic enzyme increases; however, this may not be possible with set-dose tablets.72
Zinc therapy has also been associated with significant reductions in high density lipoprotein (HDL) cholesterol (by around 20%) and total cholesterol (by around 10%) in males only. These reductions have not been found to influence cardiovascular risk,73 and no such risk was found in the overall studies presented to the EMA.31 In a paediatric cohort, the mean HDL/total cholesterol ratio significantly decreased by around 10%.49 This was hypothesised to indicate potential CD due to over-treatment.31 Lower serum cholesterol levels in hWD have been reported regardless of treatment.74 Again, further studies are needed to ascertain the relationship between zinc therapy and HDL, and cholesterol changes.
THE IMPORTANCE OF MONITORING AND TREATMENT ADHERENCE
Following treatment initiation, lifetime monitoring includes WD symptoms, serum and urinary copper, ceruloplasmin, liver enzymes, and complete blood count.18-21 With zinc therapy, 24-hour urinary copper monitoring is used to ascertain if levels are within limits (typically 30−100 µg).7,18-21,33,37,60 However, as copper is also excreted in faeces and sweat,75 urinary copper may not be an entirely accurate measure of copper levels. It remains to be ascertained if this is the best way to monitor WD treatment, why some patients develop neurological worsening or CD, and why some do not respond to treatment.
Hepatic copper levels can be obtained via liver biopsy, which is impractical beyond clinical studies,2 or via less invasive tomography/CT scans following ingestion of copper-64.27 Copper metabolism control can also be evaluated via assay of non-ceruloplasmin-bound plasma copper (typically 5−25 µg/dL); however, it is of debate as to which is the best method for determining this.20 More formal evaluation of the relationship between urinary zinc and urinary copper levels is needed, including between symptomatic and presymptomatic populations, and those with nWD or hWD.
Treatment adherence is of paramount importance in WD, with overall survival in treatment-compliant patients comparable to the general population.76 Non-adherence in pre-symptomatic patients, with either zinc or a chelator, is associated with the development of WD symptoms, including hepatic failure, and even death.76 It is thus troubling that non-adherence to zinc may be high.31,35,38-40,57
According to AASLD guidelines, non-adherence may occur in previously symptomatic patients due to ‘a false sense of security after prolonged treatment’, and in pre-symptomatic patients due to ‘incomplete buy-in for the diagnosis’.20 As such, monitoring should include 24-hour zinc excretion (typically >2,000 µg/day), along with serum levels (typically >125 µg/dL), to assess compliance.7,18-21,33,37,60 Of note, while zinc levels can help detect compliance, they may not provide a true picture, as a patient who has lapsed with taking their required zinc dose may revert to correct dosing if they know a monitoring visit is imminent, then lapse post-visit. Further investigation is needed to ascertain better ways to monitor and aid dose compliance, especially as 24-hour urine collection may be difficult for a patient.
Adherence to zinc may be hampered by gastritis,31,38,49,58-60 especially with the morning dose, treatment costs, supply issues, and treatment schedule as not all patients can take a tablet at work or school at a time, remote from their lunch break. While 50 mg 3x /day is recommended, this is partially to negate missed doses. However, in a study of healthy participants, reductions in hepatic copper content were achieved with either 150 mg 1x /day or 50 mg 3x /day ZA. Although the latter was superior to the former overall, high inter-individual differences were shown.27 Studies are needed to investigate whether such dosing is adequate to maintain copper balance and symptom control in patients with WD without increasing AEs. It may also be that fewer doses are missed with once- or twice-daily dosing, leading to better adherence and subsequent outcomes. Better guidance is needed as to how to individually ascertain what zinc dosing schedule best fits with an individual’s needs regarding symptom control, AEs, and their lifestyle.
REPRODUCTION IN PEOPLE WITH WILSON’S DISEASE
WD is associated with ovulatory and menstrual dysfunction,77,78 but for patients taking zinc, while there may be alterations in some fertility-related hormones, fertility is not necessarily affected.79 AASLD guidelines advise advanced pregnancy planning, with treatment stabilised before conceiving.20 Guidance documents point to lowering of DPA and trientine doses during pregnancy, but not of zinc.18-20 Miscarriage rates for patients with WD may be higher than the general population.80,81 However, while one study found this occurred despite zinc or DPA therapy,80 another found the relative risk to be significantly lower with these drugs.81 Offspring may be more likely to have a low birth weight, with no difference according to therapy type.80
The EMA, and some guidance documents, advise against breastfeeding while receiving zinc therapy due to potential zinc-induced CD in the infant.17,20,31 However, while zinc levels may increase in breastmilk when taking this therapy, this was apparently without consequences in one study, leading the authors to conclude that breastfeeding while taking zinc was safe.82 Despite all these findings, there are differences in guidance documents regarding zinc use during pregnancy and breastfeeding, ( Table 2) and there is a need for updates, to help guide prescribers.18-21
CONCLUSION
Zinc salts have an established role in WD treatment;18-21,33 however, the lack of head-to-head studies and randomised clinical trials limits recommendations as to where zinc should be positioned compared to chelators. With the above considerations in mind, Figure 1 highlights where there are knowledge gaps in the understanding of the use of zinc for patients with WD, and where there may be study opportunities to enhance this understanding and inform guidance documents.
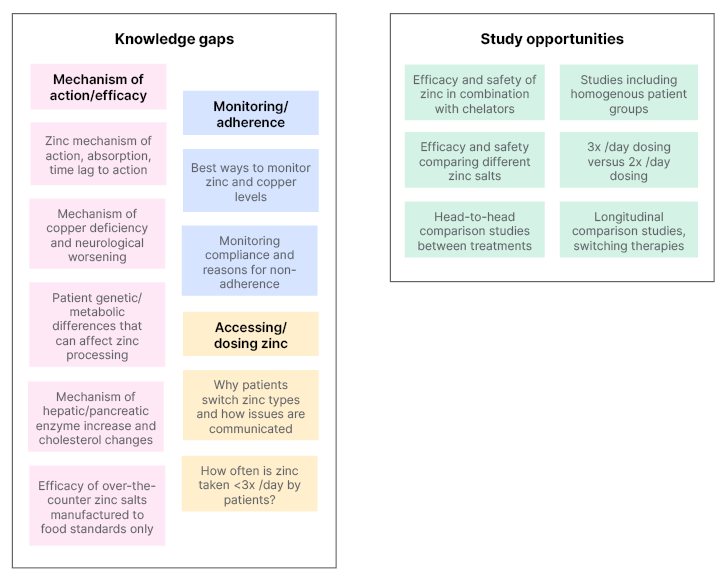
Figure 1: Knowledge gaps and study opportunities with regard to zinc use in Wilson’s disease.







