Interview Summary
Modern targeted prophylaxis is recommended for patients with hereditary angioedema (HAE), but many remain on attenuated androgens. EMJ spoke to two HAE experts who explain how they help patients to make the switch.INTRODUCTION
HAE is a rare genetic condition that affects approximately one in 50,000 people worldwide. Patients experience unpredictable episodes of cutaneous or submucosal oedema in different parts of the body, which can be life-threatening when affecting the upper respiratory tract.1 Attacks can occur without warning, and can also be triggered by a variety of factors including stress, infections, injury, and surgery.1 The unpredictable periodicity and intensity of attacks can significantly impair individuals’ quality of life (QoL).1
Most cases of HAE are caused by mutations in the SERPING1 gene that encodes the C1-esterase inhibitor (C1-INH) protein.2 The mutations result in a reduction or dysfunction in C1-INH protein (HAE-C1-INH type 1 and HAE-C1-INH type 2, respectively).2 Such mutations affect the kinin-kallikrein pathway and lead to overproduction of bradykinin, with increased vascular permeability and oedema.2
In the past decade, specific, targeted medicines for treatment of HAE attacks, and for long-term prophylaxis (LTP) to prevent attacks, have been licenced, and are now recommended in international guidelines.1 This follows decades when the only medicines available to treat HAE were non-specific compounds such as attenuated androgens (AA), which can cause debilitating side effects, and are unlicenced in many European countries.1,3
Recently available agents, including kallikrein inhibitors, such as oral berotralstat and subcutaneous lanadelumab, are transforming the way HAE can be managed, by making it possible for targeted prophylaxis to be the mainstay of their treatment.1 However, a significant proportion of patients throughout Europe and the USA are still prescribed AAs, which are not recommended as first-line prophylaxis in the guidelines.1,4-8
To find out why this is the case, and to understand what can be done to ensure more patients can access first-line prophylactics, EMJ interviewed David Launay, Department of Internal Medicine and Immunology, Hospital Centre, University of Lille, France; and Padmalal Gurugama, Clinical Immunology and Allergy, Cambridge University Hospitals NHS Foundation Trust, UK, who are both experts managing patients with HAE in specialist centres.
Both specialists are increasingly seeing patients with HAE who are keen to transition from AAs to kallikrein inhibitors. This article discusses the value of clear guidelines for treatment and management of HAE, and highlights the different approaches used to switch patients’ medications. It also provides insights that demonstrate a need for consensus among clinicians on how best to manage the transition away from AAs.
THE VALUE OF GUIDELINES FOR HEREDITARY ANGIOEDEMA MANAGEMENT
The 2021 International World Allergy Organization (WAO)/European Academy of Allergy and Clinical Immunology (EAACI) Guidelines for HAE management1 recommend long-term prophylaxis to reduce the risk of HAE attacks.1 Three medications are now licenced as first-line prophylactics: plasma-derived C1-INH and lanadelumab, which are administered subcutaneously biweekly; and berotralstat, which is an oral medication taken daily.1 Berotralstat and lanadelumab act by inhibiting the action of kallikrein by different mechanisms.1
“Prior to the guidelines,1 we were treating patients to control the number of attacks. Now the bar is set higher, and we aim to control attacks, and also to normalise their QoL. It is an important strategic change,” Launay explained.
Gurugama and Launay agree that the guidelines provide clear treatment algorithms that can be applied to all patients, in accordance with a medicine’s licencing.1 The guidelines give clinicians the confidence to talk to patients about how medication can reduce the overall burden of disease. “At all our clinics, we first ask patients to complete the Angioedema Control Test (AECT) and QoL questionnaires, recommended in the guidelines,”1 Launay said. It provides a robust way to check how well an individual’s disease is controlled, and is a good starting point for discussions about new medications.
The guidelines clearly state that AAs should only be used as second-line treatments, as they are non-specific, and have potential short- and long-term side effects,1 including an increased risk of comorbidities such as hypertension, hypercholesterolaemia, and diabetes.9 However, despite the recommendations for first-line targeted treatments, a significant number of existing patients with HAE across Europe are still being prescribed AAs.4-7 Gurugama and Launay agree that this is, at least partly, because AAs are less costly, and clinicians and patients are very familiar with these medicines. Gurugama said: “We have a cohort of more than 100 patients at our tertiary clinic, and many of them have been taking AAs for 20–30 years.” It can be difficult to convince people to change their medications.
Both specialists find the guidelines helpful when starting conversations with their patients about newly licenced and recommended treatments. The evidence-based guidelines also provide a good basis for discussions with patients currently taking AAs, who might be encouraged to transition to first-line prophylaxis.
However, according to Gurugama, who has 30–40 patients who still take the AA danazol, there is reluctance from some patients to transition away from AAs, as their disease is relatively well controlled with danazol. “Many patients who continue on AAs such as danazol are on relatively low doses, and do not experience tangible side-effects,” he explained. “Patients who still have attacks despite higher dose AAs are more open to a discussion about transitioning to first-line LTPs.”
An additional consideration for Gurugama is that, in the UK, prescribing practices are informed by the National Institute for Health and Care Excellence (NICE) guidelines.10 The NICE guidelines state that if a patient has two or more attacks per week, lanadelumab or C1-INH should be prescribed.10 If the patient has two or more attacks per month, berotralstat should be given.10 “However, these recommendations do not consider patients whose attacks are already being controlled with danazol,” Gurugama pointed out. This is also the case for the EAACI guidelines.1
Although all newly diagnosed patients receive first-line LTPs, according to Gurugama, there is an ongoing debate among some clinicians about the cost of newer targeted therapies versus attenuated androgens, which are relatively inexpensive, and can help to control attacks. This argument can be a barrier to moving patients onto first-line recommended treatments.
Launay believes the value of consensus guidelines goes beyond treatments. “The guidelines also remind us that we should talk to the patient about the overall burden of disease on their lives, rather than just the number of attacks,” he said. “This can help inform the best course of action in terms of prophylaxis,” Launay added.
CHOOSING FIRST-LINE LONG-TERM PROPHYLAXIS
There is good clinical evidence that first-line LTPs improve patient QoL.11,12 This aligns with Launay’s experience: “I see more and more patients asking for LTP because they know people with HAE that are on first-line LTPs who experience improved QoL, and they want to have that improvement in their QoL too.”
“We have several first-line LTP options, administered via different routes, and this is an advantage for patients, as they have a choice,” added Launay, who believes reducing anxiety through shared decision-making is an important part of managing HAE.
Gurugama described a recent case in their clinic. The individual was on danazol, but had not been to clinic for more than 2 years. Gurugama was able to inform the patient of new treatment options and, as a result, the individual is now transitioning to a first-line LTP.
Recent surveys of people with HAE indicate that many patients find their treatment burdensome, and would prefer to take medicines with a more convenient route of administration,13 and a majority would prefer to take an oral medication, rather than medicine administered via the intravenous or subcutaneous route.13 The convenience of an oral medication may be a factor in patients starting on LTPs.14
In Launay’s and Gurugama’s experience, the frequency of doses can also influence patient choice. “Some people are uncomfortable self-administering a subcutaneous dose,” said Launay, but others prefer to accept the burden of injectable administration, because they can take the medicine biweekly or monthly, rather than daily, making them less likely to forget. “Others may be concerned that they will forget the treatment if it is taken only once a month, so prefer a daily oral treatment,” Launay added.
ATTENUATED ANDROGEN WITHDRAWAL STRATEGIES: WHAT WORKS?
Although international guidelines for HAE management make clear recommendations on first-line treatments and prophylaxis, they do not provide clear guidance for transitioning patients from second- to first-line prophylactic medications. This is a gap that Launay and Gurugama think could be filled with further studies and real-world data analysis, and this is starting to happen.15,16
A recent survey of 12 physicians, carried out alongside a review of the endocrine literature,15 highlighted a range of approaches to androgen withdrawal. The authors conclude that there is unlikely to be a ‘one size fits all’ approach that works in every case. Many factors, such as age, gender, and coexisting conditions, can affect how an individual will respond to androgen withdrawal, both in terms of increases in attack rate, and also other adverse effects such as fatigue and mood changes, including anxiety and depression.15
“We are constantly asking ourselves ‘what is the best strategy for transitioning patients from androgens to first-line LTPs?’,” Launay said. “It is an important question, because we have a lot of patients who are taking androgens.” Launay follows approximately 200 patients at his centre and, to date, approximately 20 have transitioned successfully from androgens to first-line LTPs.
Overlapping Androgens and First-Line Long-Term Prophylaxis Before Androgen Withdrawal
When a patient decides to transition away from AAs, Launay starts them on first-line LTP while they are still taking androgens: “The medicines are effective, but do not start to work immediately. In my experience, there is a period of time before we see an effect. After that time, if the patient’s disease is controlled, the androgens are withdrawn as quickly as possible.” (Figure 1A)
Launay accepts that there may be other strategies that are useful, but reiterates that this one works: “I have not seen side effects with this approach of overlapping the medications.”
In particular, Launay is concerned about the psychological impacts for patients of experiencing an increase in attacks during the transition, and wants to avoid this, where possible: “If I stop the androgen before, or on the day I start a new treatment, the patient can experience more attacks, and this may lead them to falsely conclude that the recently introduced treatment is not working.”
Tapered Androgen Withdrawal With or Without Overlap with First-Line Long-Term Prophylaxis
Many clinicians, including Gurugama, have concerns about withdrawing androgens rapidly.15 Gurugama, who has a cohort of 30–40 patients on low-dose danazol, recommends a tapered withdrawal of AAs over a 3–4 month period (Figure 1B), with close monitoring to check for adverse effects, such as liver function abnormalities.15 Gurugama discusses the treatment plan in detail with the patient in advance, and ensures they are aware that they may experience an increase in attacks, but that they will have access to on-demand treatment to control them as soon as they arise.
“It is important the patient is aware that they may have a temporary increase in the number of attacks as androgens are withdrawn,” Gurugama said. “Patients have a good picture of what might happen and have on-demand treatment at home to provide a safety net if they experience early signs of an attack.” He added: “If my patient was on 700 mg danazol a week, I would reduce the dose by 100 mg per week. I have not seen any adverse effects using this approach.”
Also, Gurugama said, patients know that HAE is a life-long condition, and the transition away from a medication that can cause long term side-effects is important. The period when they might experience an increase in symptoms is relatively short.
Gurugama agrees that there may be some occasions where overlap with a first-line prophylactic makes sense (Figure 1B and 1C). For example, if a patient is taking AAs and still has one or two attacks per month, overlapping with berotralstat should be considered. “We believe that berotralstat has no marked effect on liver function,” Gurugama continued, but it would be good to have clinical data for overlap with low-dose danazol.
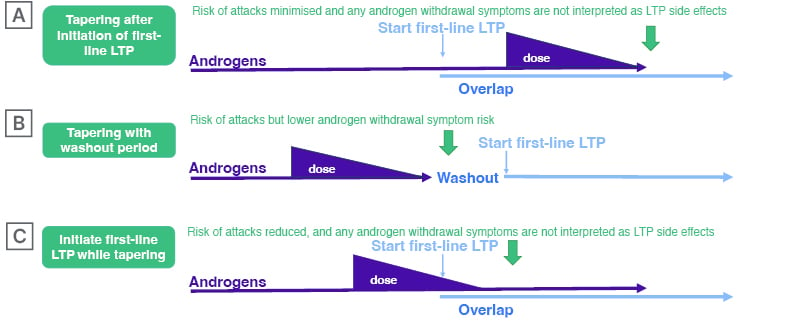
Figure 1: Possible options for transitioning to first-line long-term prophylaxis from androgens.
LTP: long-term prophylaxis.
ENCOURAGING MORE PEOPLE TO TRANSITION FROM SECOND- TO FIRST-LINE PROPHYLACTICS
The number of patients transitioning away from AAs is likely to increase dramatically in the short term, according to Launay. “Older patients are starting to get side-effects, cardiovascular disease, and other comorbidities, which were not apparent when they were younger,” explained Launay.
However, some existing patients may not be eligible for first-line LTPs, particularly in the UK. Gurugama has observed that, in a few cases, patients in their 60s and 70s remain attack-free when androgens are withdrawn: “In these cases, we can only recommend on-demand treatment, as these patients don’t meet the criteria set by NICE for treatment with first-line LTPs.10 Like many colleagues, I would like to see the NICE guidelines changed so we can prescribe first-line LTPs to all patients, as and when they are needed, because if these older individuals do go on to have further attacks, they will likely want to go back onto danazol.”
There is clear evidence from clinical trials and real-world data that licenced LTPs are more efficacious and better tolerated than AAs,1,17 and this is reassuring for patients, encouraging them to switch treatments.
To help minimise anxiety during the transition, Launay’s patients are monitored with a monthly phone call and a 3-month follow-up visit. For Launay, this close monitoring and management of the patient is critical for successful transition: “During the first weeks, patients may experience side effects and attacks, so we need to reassure them that these will diminish. It is important for them to adhere to the treatment, both in short term and long term.”
CONCLUSION
Launay and Gurugama agree better guidelines are needed to give patients and clinicians more confidence on how to transition away from AAs. Different clinicians have different experiences and it is important these are shared across the clinical community.15 “The lack of consensus on how to transition can be a reason for clinicians to avoid switching patients from second- to first-line prophylactics, because they do not know how best to do this,” Launay said.
He went on to emphasise the point, saying: “Should we recommend withdrawal or overlap? If androgen tapering works, how long should it take? When and how do we stop? We need good data.”
In addition, Launay believes guidelines that are specific for different treatments would be helpful. “It may be that a different transitioning strategy should be applied, depending on whether the patient is switching to oral or subcutaneous prophylactic medication,” Launay explained.
Gurugama agreed and concluded: “Clinicians want to provide long-term recommended prophylaxis to most of their patients. The medications are available, and we should be able to apply best practice strategies, and use them.”
| Adverse events should be reported. Reporting forms and information for the United Kingdom can be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to BioCryst UK Ltd on +44 (0)203 8850789 or email [email protected]. |
EU.HAE.00018
Date of preparation: March 2024







