INTRODUCTION
The human body is a holistic system, interconnected by the circulatory, nervous, lymphatic, endocrine, and immune systems that facilitate communication throughout the body. While these physiological highways allow distant organs to work together to perform biological functions, such as through the gut–brain and the hypothalamic-pituitary-adrenal (HPA) axes, our intricately connected network is inherently vulnerable to obstructions or perturbations, and pathogens can exploit it as a transportation system to invade or impact different parts of the body.1 Thus, maintaining these pathways free from obstructions and ensuring proper insulation of communication channels by physical and chemical barriers is essential for preserving health and preventing pathogens and their components from travelling alongside the body’s normal signals.1,2 In conjunction with these built-in crosstalks, increasing evidence suggests that the human microbiota plays an integral role in promoting human health by actively interacting with the host immune system and contributing to the development and regulation of physiological communication pathways.3,4 In this brief review, the authors discuss the interplay between the two largest human microbiome reservoirs, the oral cavity and gut, within the context of the oral–gut–brain axis and the physiological pathways these microbes could utilise to induce neuroinflammation, infiltrate the brain, and potentially contribute to the onset of Alzheimer’s disease (AD).
DISCUSSION
Living in an unsterile world, we constantly interact with bacteria, fungi, archaea, protozoa, and viruses that populate our body surfaces, these are collectively termed the microbiota.1,4,5 While the previously estimated 10-to-1 ratio of bacteria to human cells is outdated, the human body is estimated to host about one bacterium for every human cell.6 Not only are these human–microbe interactions unavoidable, they are essential, as our body actively depends on their contributions during development and throughout life. For instance, the complete absence of microbiota during a critical developmental window irreversibly impaired the HPA axis function in postnatal mice and decreased neurogenesis in adult mice.3,4 At the same time, microbial dysbiosis (i.e., alterations in microbial abundance and diversity) leads to systemic inflammation and increases the risks of diseases such as diabetes, metabolic diseases, cardiovascular diseases, colorectal cancer, and AD.1,4,5,7,8 Interestingly, a few studies reveal that taking antibiotics can reduce certain pathologies, such as amyloid-beta plaque deposition and microglial activation in AD mouse models, suggesting that transient suppression of dysbiosis or overall microbially induced neuroinflammation can improve symptoms in the brain.3-5 However, the impact of antibiotics against dysbiosis remains speculative as others have found adverse effects, such as reduced overall cognitive abilities.3-5
In line with the microbiota’s direct health impacts, faecal transplantation from healthy to diseased individuals improved cognitive health in clinical and in vivo animal studies, while the opposite outcome occurred when diseased faecal matter was transplanted into healthy mice.3,4 Thus, these studies illustrate that not all microorganisms are equal and, instead, act as a double-edged sword. Different microorganisms participate in distinct functions and produce specific molecules that lead to unique responses. Therefore, the composition and delicate balance of bacteria and other members of the microbiota (e.g., viruses and fungi) hold immense power to either induce or reverse pathology, making the study of microbiota highly complex. For this article, the authors have focused on the bacterial influence, where most of these essential players reside in the gut, which receives a continuous influx of bacteria from the oral cavity, the anatomical starting point of the gastrointestinal (GI) tract.1,9
Because microbiota are not an intrinsic part of our body but constituents of the unsterile environment, it is not surprising that the oral cavity and the gut are the twomost densely populated areas with microbes, given their direct exposure to the external environment. At the intersection of these constant microbial–host interactions is the mucosal immune system, which lines the oral cavity and GI tract, providing both physical and molecular protection against pathogenic bacteria while tolerating the commensal microbes. Under conditions of health, commensal bacteria keep the pathogenic bacterial load in check and secrete metabolites such as short-chain fatty acids (SCFA) that aid the mucosal barrier integrity.1
Moreover, IgA neutralises foreign antigens and tolerates bacterial colonisation of the mucosa while providing non-inflammatory or non-destructive protection against pathogenic invasion.10 However, microbial dysbiosis overwhelms the local immune system of the oral cavity or gut, impairing mucosal barrier integrity by causing inflammation and downregulating junctional proteins.1,9,10 Consequently, a leaky barrier develops that enables microbes to leave their residence and access the body’s communication pathways, ultimately migrating to distant organs like the liver, spleen, and brain.1,7,8 Such phenomena have been observed following periodontitis, an infection-driven, bone-resorbing inflammatory disease of the mouth that puts the body under a low-grade inflammatory state and is recognised as a risk factor for AD.1,9 Additionally, periodontal pathogens can directly induce gut dysbiosis, which is also associated with the onset and progression of AD.1,9 Hence, the impact of oral pathogens spans these separate organs, suggesting the existence of the oral–gut–brain communication axis (Figure 1).
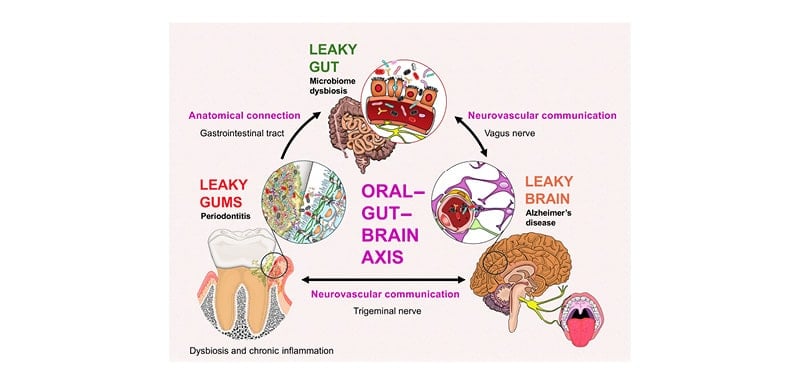
Figure 1: Pathways of communication within the oral–gut–brain axis.
Since the largest and most diverse microbiota in the body reside in the gut, the gut microbiome has been extensively studied.2-4,9 Most of the body’s immune cells are localised here, managing hundreds of trillions of microbes, and these microbe–host interactions are monitored by 100 million enteric neurons, with the vagus nerve acting as the main bidirectional communication pathway between the gut and the brain.1,3,4 This intricate communication between the microbiota, immune, and nervous systems has been characterised as the microbiota–gut–brain axis, where these microorganisms are involved in a wide array of physiological functions, including digestion.4 Emerging studies are recognising that the oral cavity is the gateway to the gut and hosts the second-largest human microbiome, with over a trillion oral bacteria reaching the gut daily via their anatomical connection.1,2,9 A comprehensive investigation of the microbial influence across the entire oral–gut–brain axis found that saliva from periodontal disease patients directly induced gut dysbiosis and impaired the intestinal barrier integrity in an AD mouse model while concurrently leading to neuroinflammation, memory deficits, and amyloid-beta plaque accumulation.9 Another study found similar results where a topically applied polymicrobial periodontal inoculum significantly increased molecular hallmarks of AD (e.g., neuroinflammation, amyloid-beta accumulation, and phosphorylated tau formation) and mice brain microbiome dysbiosis. Moreover, the same infection regimen was found to downregulate tight junctions and cause inflammation in the murine gut epithelium, as well as elevate lipid depositions in the liver.7,8 Lastly, elevated levels of periodontal pathogens have been detected in postmortem brains in patients with AD and in experimental studies, which suggest that oral pathogens can physically traverse the oral–gut–brain axis to exert inflammatory responses in both the gut and the brain (as well as other organs, such as the liver), contributing to AD pathologies.1,7
Notably, oral pathogens such as Porphyromonas gingivalis (Pg) exist in healthy individuals, albeit at low levels, and often enter the bloodstream after normal activities such as eating and brushing teeth. These pathogens can induce an inflammatory response that can influence brain functions and, in some cases, lead to atheroma formation that can obstruct the circulatory highway.1 Interestingly, the aforementioned study using the polymicrobial infection regimen detected Pg in the brains of two-thirds of the control mice, although at significantly lower DNA copy numbers than the infected group.7 Since Pg is not a natural member of the mouse microbiome, this finding raises the possibility of experimental contamination. Yet, only Pg was found in control brains and not the other three pathogens in the polymicrobial inoculum administered at equal doses. The presence of Pg in the brains of uninfected mice, despite the antimicrobial wash treatments that inhibited natural oral microbiota, raises the question of whether these pathogens in the oral cavity of healthy individuals could also exist at low levels in healthy brains, or if the presence of these oral pathogens in the brain is part of an impending disease progression.7 More studies are needed to characterise how low levels of these oral pathogens in healthy individuals influence the brain via the oral–brain axis, which is connected through the neurovascular bundles that include the trigeminal nerves, as well as the circulatory and lymphatic systems.
CONCLUSION
Nevertheless, the chronological events of oral or gut microbial dysbiosis, local inflammatory response, and barrier impairment seem to be the common thread that primes the conditions for pathogens to access our interconnected system, infecting distant organs, and leading to pathologies.9 The elderly population is particularly susceptible to this disease progression due to age-associated alteration in their microbial profile, gut-dysbiosis-induced barrier dysfunction, which leads to systemic inflammation and infection, and immunosenescence that limits their ability to combat infections.4,5,10 Remarkably, this association between ageing and increased likelihood of infections is not observed in germ-free mice, which, by design, lack the opportunity for infections and tend to live longer.4 Recognising that microbial–host interactions are an inevitable part of normal life and direct determinants of health, reshaping the dysbiotic microbiota by using prebiotics, probiotics, synbiotics, or postbiotics (commensal bacterial metabolites, such as nisin) may hold the key to achieving therapeutic efficacy.3,5,7,8 Interestingly, despite their advanced age, centenarian populations from blue zones carry distinct microbiota compositions across their bodily compartments and have superior oral health conditions compared to their corresponding controls.2 In light of this, another productive strategy may be to keep oral health in check and thereby remove one of the key contributors to gut dysbiosis and systemic inflammation, ultimately protecting both the brain and overall health along the oral–gut–brain axis.1,2,5,9







