Abstract
It is imperative to evaluate for secondary causes of membranous glomerulonephritis, from infections such as hepatitis B and C, viruses and parasites, autoimmune diseases like systemic lupus erythematosus, and other neoplasms. Tuberculosis associated with membranous glomerulonephritis is rare. The authors report a case of microbiologically proven pulmonary tuberculosis and membranous nephropathy occurring concurrently in the same patient. Antitubercular therapy alone was sufficient to cause improvement in the patient. Tuberculosis should be recognised as a potentially treatable infectious cause of secondary membranous nephropathy.
Key Points
1. A thorough evaluation always has to be done to rule out secondary causes of membranous nephropathy.2. Tuberculosis affects the kidney insidiously, and is often misdiagnosed as primary glomerulonephritis.
3. Therapy can effectively mitigate both tuberculosis and the associated glomerulonephritis.
INTRODUCTION
Mycobacterium tuberculosis causing glomerulonephritis (GN) is a rare occurrence,1 and its association has been seldom reported.2 On account of its non-specific and atypical manifestations, tuberculosis‑associated GN (TB‑GN) becomes an unusual diagnosis. The usual presentation is oedema, proteinuria, haematuria, and varying degrees of kidney dysfunction, which resemble symptoms of primary GN, thus, often leading to a misdiagnosis of primary GN rather than TB‑GN. Deterioration of renal function and the spread of TB are potentially life‑threatening complications, which occur if patients are treated with glucocorticoids or/and other immunosuppressive agents. Here, the index case the authors report is of a 20‑year‑old male with membranous nephropathy (MN) secondary to pulmonary tuberculosis (consent given).
CASE REPORT
The index case was brought to Wockhardt Hospitals, Mira Bhayandar, Maharashtra, India, in altered sensorium, and short duration (of about 1 week) history of fever and lower extremity oedema. The patient did not complain of cough with or without expectoration, or give history of weight loss. On further physical examination, a blood pressure of 90/60 mmHg, pallor, and bilateral pitting pedal oedema were found.
No palpable lymphadenopathy, skin rash, or focal neurologic deficit were documented. Laboratory analyses yielded the following findings: mild anaemia, normal white blood cell count, and mild thrombocytopenia; normal serum creatinine; urine sediment was active, i.e. 4+ protein, 10–12 red blood cells, 6–8 white blood cells per high‑power field, urine protein creatinine ratio of 7.83, and a serum albumin of 1.4g/dl; transaminitis and serositis (pleural effusion and ascites). Neuroimaging studies were normal. Cerebrospinal fluid revealed five cells per high-power field (lymphocyte predominance). Viral markers like hepatitis B or C, anti-streptolysin O, auto-immune workup (antinuclear antibodies and rheumatoid factor), anti-PLA2R antibody, and complement levels, along with serum and urine immunofixation electrophoresis and tumour markers, were all negative (Table 1). Cultures were sterile. Ultrasonography of the kidneys revealed the normal diameters with good corticomedullary differentiation, which subsequently led to the diagnosis of acute GN.
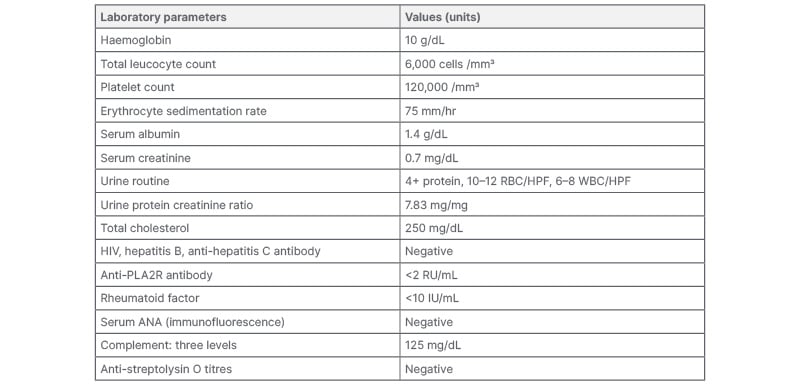
Table 1: Laboratory values: initial.
ANA: antinuclear antibodies; HPF: high-power field; RBC: red blood cell; WBC: white blood cell.
Initially, the patient was given supportive therapy and treated with lines of a non-specific viral aetiology. Once his platelet counts stabilised, a kidney biopsy was carried out. Light microscopy examination of the specimen revealed 34 mildly enlarged normocellular glomeruli with irregular thickening of basement membranes (Figure 1), with silver staining showing thickened glomerular basement membranes, with small spikes and tubules showing prominent zones of injury. The arterioles were unremarkable, and there was absence of granuloma in the renal tissue sample. Immunofluorescence revealed positive IgG staining along the glomerular capillary wall (Figure 2), with similar staining intensity of κ and λ light chains. The glomeruli did not stain for IgM, IgA, C3, C1q, IgG4, or PLA2R, and Congo red stain was negative. MN was the pathological diagnosis contemplated on the basis of the biopsy. To explore secondary causes of MN, the patient was evaluated but to no avail; hence, patient was started on angiotensin receptor blockers, and discharged in afebrile state.
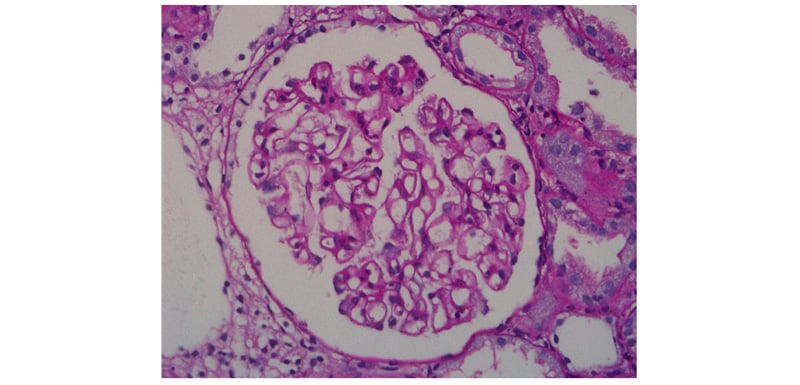
Figure 1: Periodic Acid Schiff stain showing glomerular basement membrane thickening (400x).
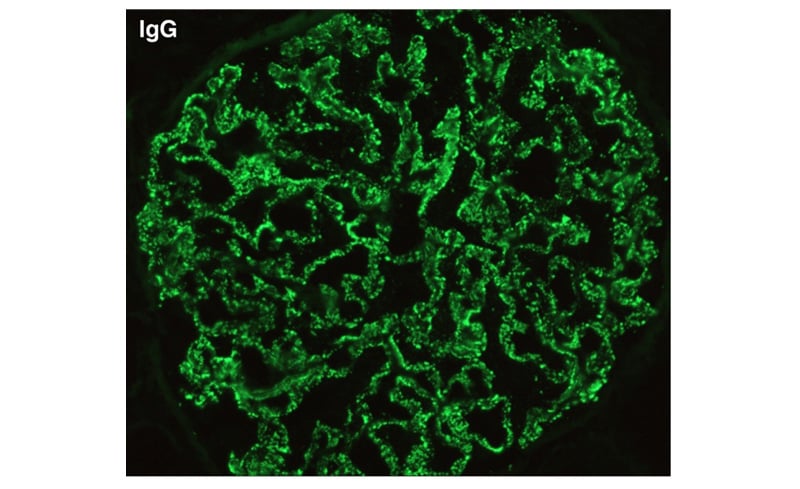
Figure 2: Immunofluorescence stain showing irregular IgG deposition (400x).
One week later, the patient again became febrile and developed hypotension; hence, angiotensin receptor blockers were stopped and he underwent PET-CT scan. This revealed a sub-lobar consolidation with cavity formation, especially in the left lower lobe; the presence of metabolically active mediastinal, subcarinal, and bilateral hilar lymph nodes with necrosis (Figure 3); and thrombo-emboli in the left upper and right lower lobe pulmonary arteries. Therefore, to confirm the aetiology, the authors also carried out bronchoalveolar lavage (BAL), which revealed M. tuberculosis by GeneXpert® (Cepheid®, Sunnyvale, California, USA; semi-nested Real time PCR).
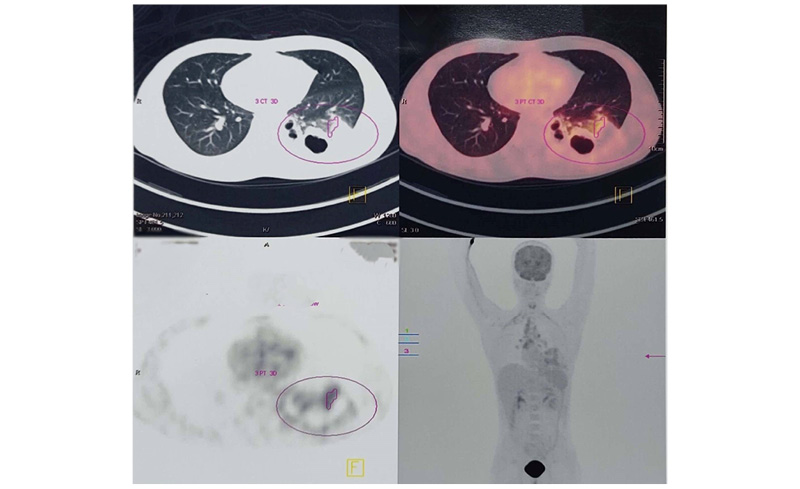
Figure 3: PET-CT scan showing sub-lobar consolidation with cavity formation and presence of metabolically active mediastinal, subcarinal, and bilateral hilar lymph nodes with necrosis.
The index case was initiated on a four‑drug anti‑tuberculosis regimen, encompassing isoniazid (H), rifampicin (R), ethambutol (E), and pyrazinamide (Z). However, the patient subsequently developed drug-induced hepatitis; hence, his drug regimen was optimised to levofloxacin (L), H, R, and E for the initial 2 months, followed by H, R, and E for the subsequent 4 months, along with oral anti-coagulation targeting a PT-INR of around 2.5. After 2 months of treatment, the patient’s urinary protein creatinine ratio reduced to 0.5, and serum albumin increased to 3.5 g/dL.
One week later, the patient again became febrile and developed hypotension; hence, angiotensin receptor blockers were stopped and he underwent PET-CT scan. This revealed a sub-lobar consolidation with cavity formation, especially in the left lower lobe; the presence of metabolically active mediastinal, subcarinal, and bilateral hilar lymph nodes with necrosis (Figure 3); and thrombo-emboli in the left upper and right lower lobe pulmonary arteries. Therefore, to confirm the aetiology, the authors also carried out bronchoalveolar lavage (BAL), which revealed M. tuberculosis by GeneXpert® (Cepheid®, Sunnyvale, California, USA; semi-nested Real time PCR).
The index case was initiated on a four‑drug anti‑tuberculosis regimen, encompassing isoniazid (H), rifampicin (R), ethambutol (E), and pyrazinamide (Z). However, the patient subsequently developed drug-induced hepatitis; hence, his drug regimen was optimised to levofloxacin (L), H, R, and E for the initial 2 months, followed by H, R, and E for the subsequent 4 months, along with oral anti-coagulation targeting a PT-INR of around 2.5. After 2 months of treatment, the patient’s urinary protein creatinine ratio reduced to 0.5, and serum albumin increased to 3.5 g/dL.
DISCUSSION
As per the information currently available, membranous pattern of glomerular injury is unique in a patient with TB, and, so far, has been seldomly documented in the literature. Based on the available clinical data and investigations, secondary causes like medication use, neoplasms, autoimmune diseases, and viral hepatitis were all winnowed out. A clue to suspect TB was offered by the abnormal findings of PET-CT and non-resolving fever. Hence, a final diagnosis of TB leading to the development of MN was considered, based on the positive BAL sample’s detection of M. tuberculosis by PCR method, and the index case’s favourable response to anti‑TB treatment, which, in turn, demonstrates the cause-and-effect relationship between TB and the glomerular injury. GN associated with TB often tends to complicate the treatment in terms of the sequence and modality of therapeutic options, i.e., anti‑TB drugs and/or immuno‑suppressive agents. The latest available information has revealed GNs of various forms which are associated tuberculosis to show inconsistent responses to several available treatment options.3 Anti‑tuberculosis drugs reduce the antigen load, which in turn reduces immune complex formation, all by decreasing the mycobacterial load from circulation.4
In the index case, the authors’ patient presented with nephrotic syndrome, and normal kidney function tests, but was found to have active M. tuberculosis in BAL; hence, immunosuppression was not considered. Treatment for tuberculosis itself provided favourable results by causing amelioration of proteinuria and symptoms; hence, the aetiologic link was further strengthened. Solak et al.1 identified 15 cases of GN associated with TB. Of these, six cases of IgA nephropathy were noted: four of crescentic GN; two mesangio-proliferative; and one each of collapsing GN, mesangio-capillary GN and membranous GN.1 A case of MN and TB has also been published in Japanese literature, but it was a diagnosis of renal TB.5
CONCLUSIONS
To summarise, based on the authors’ index case, several points need to be emphasised. Firstly, a thorough evaluation should be done in MN to rule out secondary cause, especially in patients at either end of the age spectrum. Secondly, TB affects the kidney insidiously, and is often diagnosed as de novo or primary GN due to its indistinct manifestations. Thirdly, therapy for treatment of TB can effectively mitigate both TB and the associated GN. Lastly, prior to commencement of any therapy, kidney biopsy acts as an important tool in securing a precise diagnosis.







