Interview Summary
Methotrexate is a common first-line treatment for rheumatoid arthritis, yet its widespread and habitual usage often leads physicians to overlook the choice of administration route when planning management strategies. A recent survey involving 30 consultant rheumatologists from France, Germany, Italy, Poland, Spain, and the UK, identified variation in the utilisation and perceptions regarding oral versus subcutaneous delivery for methotrexate. In November 2023, EMJ interviewed Roberto Caporali, Professor of Rheumatology at the University of Milan, and Head of the Department of Rheumatology and Medical Sciences at Gaetano Pini Hospital, Milan, Italy. Caporali’s expertise is in clinical practice, teaching, and research in rheumatology, mainly rheumatoid arthritis and other immune-mediated inflammatory diseases, with a focus on prognostic factors, biomarkers, and treatment for patients with moderate-to-severe active rheumatoid arthritis. During this interview, Caporali discussed the decision-making process for treating rheumatoid arthritis, with a particular focus on the use of methotrexate. The purpose was to gain insights from a rheumatology expert regarding the prevalence and management goals of the disease, and available treatment options. The interview considered key decision-making drivers and barriers to healthcare professionals when selecting the route of administration. Caporali suggested that the efficacy and safety profile of methotrexate when delivered subcutaneously may be the optimal choice for patients, often resulting in higher adherence compared to oral dosing. Caporali recommended education and re-evaluation of local guidelines to improve patient outcomes by better understanding the optimal use and efficacy of methotrexate.THE ROLE OF METHOTREXATE IN THE TREATMENT OF RHEUMATOID ARTHRITIS
Rheumatoid arthritis is a chronic disease that causes pain and inflammation of the joints. Methotrexate is a synthetic disease-modifying antirheumatic drug (DMARD) that is considered the ‘anchor drug’ for rheumatoid arthritis because of its favourable efficacy, safety profile, and low cost.1,2
The mechanism by which methotrexate reduces disease activity and controls the progression of rheumatoid arthritis is not well understood. Methotrexate may act through several pathways, such as folate antagonism, extracellular adenosine signalling, generation of reactive oxygen species, decrease in adhesion molecules, alteration of cytokine profiles, and interaction with T and B lymphocytes.3 Studies also suggest that methotrexate may suppress the JAK-STAT pathway.4
Methotrexate is the recommended first-line treatment for rheumatoid arthritis.2,5-7 The American College of Rheumatology (ACR) and European Alliance of Associations for Rheumatology (EULAR) guidelines recommend methotrexate monotherapy, or in combination with low-dose short-term glucocorticoids.2,5 It should be noted that methotrexate is contraindicated in patients with renal impairment or early intolerance.5
It is important to tightly control rheumatoid arthritis at the beginning of the disease. For adults, early diagnosis and the ‘treat-to-target’ strategy are crucial in achieving remission or low disease activity.2 Caporali considered treat-to-target as “the most important strategy” for patients with rheumatoid arthritis. He also stated that the “window of opportunity” to diagnose and treat the patient is approximately 90 days, with check-ups every 1–3 months, a response expected within 3 months, and achievement of remission within 6 months.2,6,7
PATTERNS OF METHOTREXATE USE FOR RHEUMATOID ARTHRITIS: TREATMENT VARIATIONS ACROSS EUROPE
Caporali outlined a web-based survey that was conducted across six European countries (France, Germany, Italy, Poland, Spain, and the UK), to understand the patterns of methotrexate use. The survey included 30 consultant rheumatologists (Accord Healthcare, data on file UK-05568) and examined the drivers of decision-making, and barriers influencing the choice of route of methotrexate administration.
The survey identified differences across the European countries, reporting that 42% of patients with rheumatoid arthritis on average were using oral methotrexate, while 58% of patients were using subcutaneous methotrexate. The UK demonstrated the highest first-line oral methotrexate use (94% of patients), and Poland showed a preference for first-line oral administration (68% of patients). When excluding the UK data, on average, 66% of patients use subcutaneous methotrexate compared to oral use (34% of patients initiated first-line oral [Accord Healthcare, data on file UK-05568]). Italian rheumatologists preferred subcutaneous administration, with the highest rate of first-line subcutaneous methotrexate use (94% of patients), followed by Germany (83% of patients), and Spain (58% of patients; Figure 1 [Accord Healthcare, data on file UK-05568]).
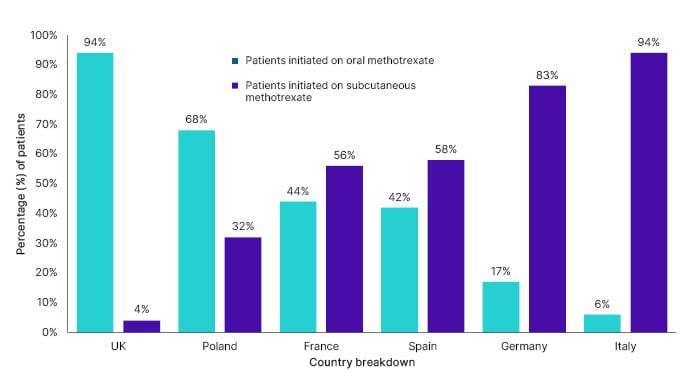
Figure 1: Percentage of patients initiated onto oral versus subcutaneous methotrexate as first-line treatment, by country (Accord Healthcare, data on file UK-05568).
Influencing Factors on Methotrexate Choice in Rheumatoid Arthritis Treatment
According to the survey, the majority of rheumatologists perceived the route of methotrexate administration as important, and both options allow them to individualise treatment.
Caporali suggested that in the UK, the high prevalence of oral methotrexate is in line with the local National Institute for Health and Care Excellence (NICE) guidelines, which recommend oral methotrexate as the primary first-line option.6,7 This may also be due to the lower cost of oral methotrexate compared to the subcutaneous form. The EULAR guidelines recommend considering the individual, medical, and societal costs associated with rheumatoid arthritis.5 The NICE guidelines consider both cost-effectiveness and a treat-to-target strategy when making recommendations.6,7 When two equally suitable treatments are available for a particular patient, the less expensive option is favoured.5-7 This recommendation also extends to the choice between oral and subcutaneous methotrexate.2,5 While the EULAR guidelines do not specify the most suitable form of methotrexate administration,5 the ACR recommends starting with oral administration, and then switching to subcutaneous if the target is not achieved.2
Caporali noted that the cost difference between oral versus subcutaneous is relatively small in contrast to the overall rheumatoid arthritis treatment cost, suggesting this should be a consideration for guidelines. Utilising subcutaneous methotrexate earlier may delay the transition to more expensive biologics.8
The survey found that Italian rheumatologists prioritise subcutaneous methotrexate administration from the outset of the disease, which was attributed to perceived efficacy (identified by 67% of consultant rheumatologists from all countries); better adherence; and improved facilities for self-injection, with the easy-to-use auto-injectors availability (Accord Healthcare, data on file UK-05568). Caporali noted the subcutaneous delivery injectors enabled patients to receive the exact and correct dose.
Caporali also asserted that subcutaneous delivery is “more active than the oral administration,” advocating for its early use.9 He suggested subcutaneous administration may be more effective due to higher bioavailability, whereas oral administration may be less effective for medium to high methotrexate doses (more than 15 mg per week).9 Caporali also suggested that patient adherence to subcutaneous administration may be higher compared to oral use.10 Put simply, Caporali said: “One subcutaneous injection once per week compared to six to eight pills,” for 15 mg or 20 mg doses, respectively, may be preferred by patients.
Patient Preference on Route of Methotrexate Administration
Caporali emphasised that when considering route choice, physicians often neglect to question the real difference between the two options, overlooking factors such as efficacy and bioavailability. He stated that physicians tend to prioritise perceived patient preference when selecting between oral and subcutaneous methotrexate.
The survey identified that patient preference was a major factor for oral methotrexate. However, this preference could be influenced by healthcare professionals who recommend oral methotrexate based on their own perceptions. After all, it is understandable that most patients would favour an oral tablet over an injection. Nevertheless, this perspective does not take into account potential differences in outcome benefits for patients in terms of efficacy, tolerability, and even pill burden experienced by patients. However, patient preference did not appear to be a major factor in the choice of administration route in the UK (Accord Healthcare, data on file UK-05568).
Caporali identified a potential lack of education among physicians regarding methotrexate use, particularly among newer physicians who may prescribe almost ‘automatically’ based on local practices, rather than thoughtful consideration. Caporali believed newer rheumatologists exhibit more awareness and curiosity for more modern alternatives, such as biologics.
Caporali further contended that patient adherence to treatment is likely to improve when patients are comfortable with the chosen drug administration method from the outset. Additionally, he also addressed patient misconceptions, refuting misinformation sourced from Internet searches, and providing accurate information about the true nature and efficacy of methotrexate in rheumatoid arthritis. Methotrexate was originally used as a cancer treatment; some patients may hold the misconception that they are receiving high-dose anti-cancer chemotherapy, and the risks associated with this, rather than a DMARD. Caporali emphasised the significance of dispelling this misconception at the earliest opportunity.
SIDE EFFECTS ASSOCIATED WITH THE ROUTE OF METHOTREXATE ADMINISTRATION
Caporali noted that subcutaneous administration has a preferable tolerability profile, presenting fewer gastrointestinal side effects.9 This was supported by the survey findings, where 73% (n=22) of consultant rheumatologists cited that subcutaneous injections were associated with fewer side effects. He stated that subcutaneous administration may be more favourable for patients due to reduced incidence of gastrointestinal complaints, whereas oral methotrexate may be preferable to those averse to injections. Despite needle phobia (cited by 47% [n=14] of rheumatologists) and injection site reactions (cited by 27% [n=8] of rheumatologists) being the main disadvantages to subcutaneous use, Caporali noted that, in Italy, patients who avoid subcutaneous use due to needle phobia or self-injection are, in his opinion and experience, minimal (fewer than 10%).
Caporali identified that other side effects, including transaminase elevation and flu-like symptoms, were noted to be similar between the two administration methods.9 To address gastrointestinal side effects, the ACR recommends adjusting the dose, or increasing the folic acid intake.2
SWITCHING DYNAMICS BETWEEN ROUTES OF METHOTREXATE ADMINISTRATION
The survey found that 26% of patients who started on oral methotrexate switched to subcutaneous methotrexate, due to issues with low efficacy and tolerability, such as nausea and gastrointestinal complaints. The timeframe for switching typically occurs within 3–6 months, as per guidelines,2,6,7 but can be longer in the UK (Accord Healthcare, data on file UK-05568). Caporali explained that the UK had a longer timeframe for switching, whereas other European countries tended to react faster to treatment switching. The prevalence of switching from oral to subcutaneous was particularly prevalent in France (48% of patients) and Poland (41% of patients [Accord Healthcare, data on file UK-05568]).
Conversely, very few patients switched from subcutaneous to oral methotrexate (12% of patients), with patient request being the primary reason (Accord Healthcare, data on file UK-05568). Of note, very few patients reverted back to oral methotrexate after switching to subcutaneous (1–2% of patients [Accord Healthcare, data on file UK-05568]).
Caporali noted that, in his experience, switching from subcutaneous to oral is rare, and usually only due to injection site reactions, which are uncommon. In cases where patients do not show improvement with oral methotrexate, Caporali recommended switching to subcutaneous methotrexate instead of another conventional DMARD or biologic, as recommended by the ACR Guideline.2
FUTURE DIRECTIONS AND EDUCATIONAL NEEDS FOR METHOTREXATE USE
Caporali emphasised that patient education is essential in addressing misconceptions about methotrexate use. He also identified the importance of early communication with patients about their rheumatoid arthritis diagnosis and treatment, particularly regarding methotrexate use.2 There is a need, he went on, to improve rheumatologist awareness and education regarding the similarities and differences in efficacy, safety, and bioavailability between oral and subcutaneous methotrexate administration. Caporali believes this should be a major driver of choice, which is currently not the case.
Caporali also noted that the longstanding and frequent use of methotrexate means physicians often prescribe automatically, without stopping to consider the route of administration. For example, NICE guidelines may drive choice in the UK, but they do not consider efficacy and patient preference. The challenge lies in motivating physicians to engage with educational initiatives, as they may believe they possess sufficient knowledge of a drug that has been widely used since its introduction in the 1950s, said Caporali. Addressing this challenge is crucial for ensuring informed and deliberate prescribing practices among healthcare professionals, he added.
Caporali also identified that guidelines should provide clearer recommendations on dosage adjustments, managing side effects, and transitioning between administration routes. Further research is needed to evaluate the effectiveness and safety of different strategies, such as dose reduction or discontinuation, in patients with long-standing remission. The challenge, however, lies in raising questions that make physicians realise they may not know as much as they thought about this highly used and long-established therapy.
SUMMARY
Caporali believes education and rethinking around methotrexate and route of administration choice are necessary to prompt changes in guidelines, optimise treatment, and improve patient outcomes. Re-evaluating and updating local guidelines may be necessary to better reflect evidence on efficacy and optimal methotrexate use. Education of both rheumatologists and patients is important to optimise methotrexate treatment decisions and adherence.
All adverse events should be reported. Reporting forms and information can be found in your local country. Adverse events should also be reported to Accord using the contact details dependant on the country you are based.
UK/IE: Accord UK-LTD on +44 (0) 1271 385 257 or email [email protected].
Rest of EU: [email protected].
UK Adverse events reporting forms and information can also be found at www.mhra.gov.uk/yellowcard.
UK-05505 Date of preparation: November 2023







