Abstract
Background: Extrapulmonary tuberculosis (EPTB) diagnosis is difficult due to its subclinical or nonspecific clinical symptoms, paucibacillary nature, and difficulties in obtaining qualified pathological specimens for Mycobacterium tuberculosis detection. Given the paucibacillary nature of EPTB, and drug resistance as high as 19%, rapid diagnostic methods like Xpert MTB/RIF (Cepheid, Sunnyvale, California, USA) can make a significant clinical impact. In this study, the authors aim to determine the positivity rate of Mycobacterium tuberculosis in EPTB samples by Xpert MTB/RIF or cartridge-based nucleic acid amplification test (CBNAAT) in a tertiary healthcare setup.
Materials and methods: This was a retrospective cross-sectional study where presumptive extrapulmonary cases of any age were considered for inclusion. A total of 688 suspected extrapulmonary samples were analysed by CBNAAT over a period of 7 months.
Result: A total of 25% (170/688) of the cases were reported to be positive, while the combined rate of Error, Invalid, and No result was 8.5% (59/688). Rifampicin resistance was seen in 14 isolates, while four isolates showed indeterminate results.
Conclusion: Varied clinical presentations and the paucibacillary nature of the extrapulmonary samples often lead to the failure of diagnosis by conventional diagnostic tests (smear microscopy and culture). Hence, molecular diagnostic techniques play a crucial role in rapid diagnosis. The present study thus highlights the impact of CBNAAT in the definite diagnosis of extrapulmonary tuberculosis in a tertiary healthcare centre.
Key Points
1. Extrapulmonary tuberculosis (EPTB) poses significant diagnostic challenges due to its diverse clinical presentations. With tuberculosis remaining a major global health concern, timely and accurate diagnosis of EPTB is critical to improving patient outcomes and reducing disease burden2. This study evaluates the performance of the cartridge-based nucleic acid amplification test (CBNAAT) assay in diagnosing extrapulmonary tuberculosis across various specimen types in a high-burden tertiary care centre in India.
3. CBNAAT is an effective diagnostic tool for EPTB, providing rapid and reliable results. However, resource constraints and methodological limitations highlight the need for continued refinement and integration with other diagnostic strategies in resource-limited settings.
INTRODUCTION
Tuberculosis (TB) represents a significant global public health issue, ranking as the 13th leading cause of mortality and standing as the second most lethal infectious disease globally, following COVID-19. It poses a significant diagnostic and therapeutic challenge globally. India accounts for around 21% of the TB incidence.1 Mycobacterium tuberculosis (MTB) primarily infiltrates the lungs, giving rise to tuberculous lesions recognised as pulmonary TB. However, there are instances where it may infrequently invade other locations as well. Extrapulmonary tuberculosis (EPTB) is characterised, according to WHO classification criteria, as an infection caused by MTB that impacts tissues and organs outside the pulmonary parenchyma.2 In 2019, extrapulmonary tuberculosis (EPTB) cases constituted 15% of the 7.2 million reported cases of TB worldwide.3 In India, EPTB makes up 10–15% of the total TB cases, mainly affecting the pleura, lymph nodes, gastrointestinal tract, and various other organs, with a notable case mortality rate ranging from 25–50%.4 Individuals with compromised immune systems and young children exhibit an increased prevalence of EPTB.5-7
In resource-limited settings, the primary method for diagnosing TB is still smear microscopy. In highly prevalent bacillary diseases like cavitary TB, its sensitivity is approximately 50%, but it decreases to 10–20% for paucibacillary disease.8-10 Microbiological culture of MTB stands as the gold standard test; however, obtaining a complete culture result takes a minimum of 10 days in liquid media and up to 8 weeks on solid media.9-11 Additionally, there is an added delay of 3–4 weeks for drug susceptibility testing.12
Diagnosing EPTB proves challenging because of its subclinical or nonspecific clinical symptoms, paucibacillary nature, and the challenges in obtaining adequately qualified pathological specimens for detecting MTB. The delayed diagnosis of EPTB may result in untimely treatment and subsequent severe consequences.
To address the challenges associated with MTB diagnosis, in December 2010, the WHO endorsed the use of cartridge-based nucleic acid amplification test (CBNAAT)/GeneXpert MTB/RIF1 (Cepheid, Sunnyvale, California, USA) in TB laboratories. India incorporated CBNAAT into its Revised National Tuberculosis Control Program (RNTCP) in 2012.13 The CBNAAT assay employs a closed system based on real-time polymerase chain reaction (PCR), requiring minimal technical expertise for the diagnosis of TB and rifampicin resistance within 2 hours.14 In 2013, WHO updated its policy statement, endorsing the use of Xpert MTB/RIF instead of conventional microscopy and culture as the initial diagnostic tests for all adults and children suspected of having pulmonary TB. Furthermore, in 2014, WHO recommended CBNAAT over conventional tests (including microscopy, culture, or histopathology) for examining specific non-respiratory specimens (such as lymph nodes and other tissues) from patients suspected of having extrapulmonary TB.15
Considering the paucibacillary nature of EPTB and the prevalence of drug resistance reaching as high as 19%, the implementation of rapid diagnostic methods like Xpert MTB/RIF can have a substantial clinical impact.16,17 In this study, the authors aim to determine the positivity rate of MTB in EPTB samples by Xpert MTB/RIF or CBNAAT in a tertiary health care setup.
METHODS
The research conducted was a retrospective cross-sectional study spanning 7 months, from January–August 2022. A total of 688 suspected extra-pulmonary samples were collected. Presumptive EPTB cases of any age were considered for inclusion. All samples, received in sterile containers, were aseptically collected specimens, typically devoid of other microorganisms (sterile). These specimens included fluids such as spinal, pleural, pericardial, synovial, ascitic, blood, tissues (lymph node, tissue biopsies), fine needle aspirates, and infected specimens (gastric lavage, bronchial washings, pus). In the initial process, samples were concentrated at 3,000 g for 15 minutes, and sediments were then resuspended in 2–5 mL of sterile phosphate buffer saline for another 15 minutes (phosphate-buffered saline).18 Contaminated samples underwent decontamination with N-acetyl-cysteine sodium hydroxide (NALC-NaOH) before centrifugation. Biopsy samples were cut into small pieces with a sterile scalpel or blade and homogenised in sterile saline. Fine needle aspirate samples were collected by a pathologist, while other body fluid samples were collected by physicians during patient investigations and sent to the Directly Observed Therapy Centre for microscopic investigation and analysis through the GeneXpert MTB/RIF assay. For the GeneXpert MTB/RIF assay, samples with sufficient volume were treated with sample reagent containing NaOH and isopropanol, following the manufacturer’s instructions.19.20
Sample Size Calculation
This retrospective study utilised the available data or cases within a specified time period (7 months); hence, the total number of available samples during the defined study period (688 samples) was used as the sample size. All the collected samples were considered for inclusion in the study, provided they met the study’s inclusion criteria and were suitable for analysis. Any specific exclusion criteria or limitations in the data were carefully considered to ensure the integrity and representativeness of the sample.
Ethical Consideration
The retrospective nature of this study utilised existing data collected as part of routine investigation protocol; hence, no formal ethical consent was required/sought. However, adherence to ethical principles was ensured throughout the study, including the protection of patient confidentiality and privacy. All data were handled securely and anonymised to maintain patient anonymity. The study complied with relevant data protection and sought appropriate permissions for data access and use.
Data Management and Statistical Analysis
The data were recorded using the Microsoft Excel (Redmond, Washington, USA) spreadsheet program. Categorical variables were analysed using frequencies and percentages. Graphical representation, including data and bar/pie charts, was employed for appropriate data visualisation.
RESULTS
In this study, a total of 688 suspected extra-pulmonary samples were analysed over a period of 7 months. The age of the patients ranged from 1.5 months to 88 years, with a mean age of 27.49±16.82 years.
A total of 25% (170/688) of the cases were reported to be positive, while the combined rate of Error, Invalid, and No result was 8.5% (59/688). Rifampicin resistance was seen in 14 isolates, while four isolates showed indeterminate results.
Table 1 depicts the comprehensive overall distribution of the specimen types among the extra-pulmonary samples. A total of 351 pus, 155 cerebrospinal fluid, 56 pleural fluid, 51 ascitic fluid, 14 knee and synovial fluid, 34 tissue biopsy, and 27 lymph node biopsy samples were tested by CBNAAT. Table 2 and Table 3 depict the age and site-wise distribution of positivity of extrapulmonary TB among females and males, respectively.

Table 1: Percentage-wise distribution of the specimen types of extra-pulmonary samples (n=688).
CBNAAT: Cartridge-based nucleic acid amplification test; CFS: cerebrospinal fluid.
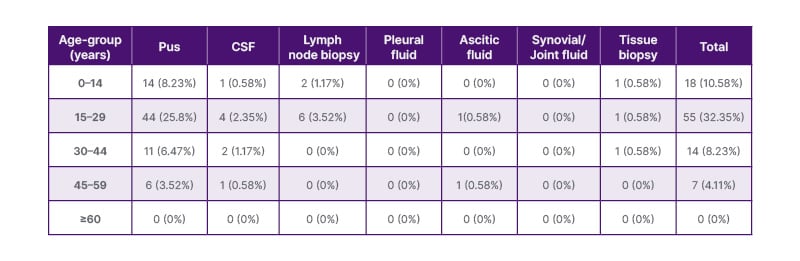
Table 2: Age and site-wise distribution of positivity of extrapulmonary tuberculosis in females (n=170).
CFS: cerebrospinal fluid.
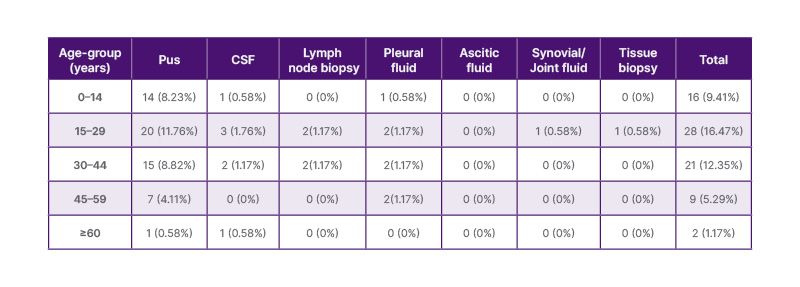
Table 3: Age and site-wise distribution of positivity of extrapulmonary tuberculosis in males (n=170).
CFS: cerebrospinal fluid.
DISCUSSION
Both developed as well as developing countries have seen an increase in the burden of TB over the past few decades, and a huge proportion of this surge is associated with the HIV epidemic. Disease pattern has evolved with the increased emergence of EPTB and disseminated TB. Drug resistance is a significant issue, especially for people with EPTB and HIV coinfection.21 Diagnosis of EPTB is rather challenging due to the paucibacillary nature of the samples.22 The WHO endorsement of GeneXpert particularly applied to the diagnosis of pulmonary TB because it was created and optimised for testing sputum samples, and the original large-scale evaluations were performed on patients with pulmonary TB only. However, evaluations of the GeneXpert assay have more recently been extended to a range of extrapulmonary samples.23 This study reported an overall positivity rate of 25% by CBNAAT for extrapulmonary samples, indicating a significant burden of MTB infection among suspected EPTB cases. Similar results were observed in a study conducted by Nishal Net al.24 focusing on the diagnostic yield of CBNAAT in the diagnosis of EPTB. In this study, CBNAAT yielded positive results in 30.76% of EPTB cases.24 It is noteworthy that the combined rate of Error, Invalid, and No result was 8.5% (59/688 cases). These results suggest that while the CBNAAT assay is a valuable tool, there are still challenges associated with the accuracy and reliability of the test. Further investigation and quality control measures are necessary to address these limitations and improve the overall performance of the assay. The presence of rifampicin resistance in 14 isolates raises concerns about drug-resistant strains of MTB in the extra-pulmonary setting. This finding highlights the importance of detecting drug resistance early to guide appropriate treatment strategies and prevent the spread of resistant strains.
Table 1 provides a detailed analysis of the performance of the CBNAAT assay and the characteristics of MTB infection across various sample types. Pus samples, which comprised the largest proportion of the specimens (351 samples), exhibited a relatively high positivity rate of 37.6% (132/351 samples). This suggests that pus samples are a valuable source for detecting MTB in extra-pulmonary cases. However, it is important to note that a significant number of samples (26/351) resulted in Error, Invalid, or no DNA findings. This indicates technical challenges or issues related to the quality of the samples or the CBNAAT assay process. Among the ascitic fluid samples (51 samples), the CBNAAT assay detected MTB in only two cases (3.92%). It is noteworthy that three samples yielded Error, Invalid, or no DNA results. This suggests potential limitations in the sensitivity of the assay for detecting MTB in ascitic fluid, or challenges associated with the collection and processing of these particular samples.
Lymph node biopsy samples (27 samples) demonstrated the highest positivity rate among all specimen types, with 44.44% (12/27 samples) testing positive for MTB, which is in concordance with the findings of another study done on EPTB.24 A study done by Kumari et al.25 on the correlation of tubercular lymphadenopathy with Ziehl-Neelsen staining also had similar findings. In the authors’ study, three samples resulted in Error, Invalid, or no DNA findings, and two samples showed rifampicin resistance for lymph node biopsy. These findings suggest the need for careful interpretation of results and further investigation into assay performance in lymph node biopsy samples. TB lymphadenitis can be difficult to diagnose since it mimics several other infections, including leprosy, sarcoidosis, and fungal infections.21 Diagnosis of TB lymphadenitis relies on fine-needle aspiration cytology, a less invasive technique than excision biopsy.26
For pleural fluid samples (56 samples), the CBNAAT assay showed a positivity rate of 12.5% (7/56 samples). The positivity of pleural TB ranged from 3–25%, as reported in many studies.24 Similar to ascitic fluid samples, a subset of pleural fluid samples (3/56) resulted in Error, Invalid, or no DNA findings. This indicates the need for further investigation to understand the factors influencing assay performance in pleural fluid specimens. All patients with an unidentified pleural effusion should be evaluated for tuberculous pleuritis.
Cerebrospinal fluid (CSF) samples, the second-largest group with 155 samples, exhibited a positivity rate of 9.67% (15/155 samples). Interestingly, 15 CSF samples were associated with Error, Invalid, or no DNA results, suggesting potential challenges or limitations in detecting MTB in CSF samples using the CBNAAT assay. Additionally, three CSF samples yielded indeterminate rifampicin resistance results, indicating the complexity of interpreting assay outcomes in this specimen type. TB meningitis is the most lethal form of meningitis and, if left untreated, almost always results in death. Delaying therapy frequently results in long-term neurological complications.21 The diagnosis of TB meningitis is a challenge owing to the difficulty in obtaining a sufficient volume of CSF sample, especially in cases of paediatric population.21,27 Joint/synovial fluid samples (14 samples) exhibited a lower positivity rate of 7.14% (1/14 samples). Two samples in this category were reported as Error, Invalid, or no DNA. The relatively low number of positive cases suggests that joint/synovial fluid may not be the primary specimen type for detecting MTB in extra-pulmonary cases. Osteoarticular tuberculosis accounts for around 10–15% of EPTB.28 While infections can occur in any bone or joint, but the spine, hip, and knee account for 70–80% of the infections.21 In the developing world, osteoarticular tuberculosis is a significant issue and one of the leading causes of osteomyelitis.29 Due to deep, inaccessible lesions, detection of osteoarticular TB is challenging.21 Early diagnosis and prompt initiation of antitubercular therapy are essential in these situations because delay results in irreversible joint damage, permanent disability of various degrees, and may also cause kyphosis and neurological complications.21,29
A comparison between Table 2 and Table 3 reveals some interesting patterns. Overall, females had a higher proportion of positive cases in each age group compared to males. The age group of 15–29 years showed the highest positivity rate in both males and females. These findings suggest that certain age groups, particularly adolescents and young adults, may be more susceptible to extrapulmonary TB infection. Among the specimen types, pus samples consistently had the highest positivity rates in both genders and across different age groups. Pus samples appear to be a reliable specimen type for detecting MTB in both males and females. CSF and lymph node biopsy samples also demonstrated significant positivity rates, highlighting their importance in the diagnosis of extrapulmonary TB, especially in paediatric cases. It is important to note that the differences observed between males and females could be influenced by various factors, including differences in immune response, exposure to risk factors, and healthcare-seeking behaviour. Further research is warranted to explore these gender-related differences and their implications for TB diagnosis and management.
Recent advancements in the field of tuberculosis diagnostics have brought attention to the potential of molecular techniques.30 Notably, studies in the current literature emphasise the growing significance of nucleic acid amplification tests, such as CBNAAT, in the accurate and rapid diagnosis of EPTB. These molecular methods have demonstrated superior sensitivity, particularly in cases with paucibacillary nature, where conventional methods often fall short.31 The findings align with the present study, affirming the efficacy of CBNAAT in enhancing diagnostic capabilities for EPTB.
Moreover, the ongoing research landscape underscores the importance of adopting innovative diagnostic approaches for tuberculosis, given the dynamic nature of the disease and the rising concern of drug resistance. The integration of CBNAAT into routine diagnostic protocols has proven instrumental in addressing these challenges, offering a timely and precise method for detecting both pulmonary and EPTB.30 As the global health community continues to combat the tuberculosis burden, incorporating cutting-edge diagnostic technologies like CBNAAT becomes paramount for achieving accurate diagnoses and initiating prompt therapeutic interventions.
CONCLUSION
In conclusion, this study provides valuable insights into the performance of the CBNAAT assay and the characteristics of MTB infection in different specimen types. It highlights the importance of considering age, gender, and specimen type when diagnosing EPTB. Future efforts should focus on refining diagnostic techniques, ensuring quality control measures, and developing strategies to combat drug resistance in EPTB cases.
Limitations
This study has several limitations. First, as CBNAAT is currently the frontline diagnostic test under the National Tuberculosis Elimination Program (NTEP) in India, the authors did not include a comparison with conventional diagnostic methods for all cases. This is due to both the limited availability of these resources and the high patient volume at their tertiary care centre, which primarily relies on CBNAAT for timely and efficient diagnosis of EPTB.
Second, while CBNAAT provides rapid and specific detection of MTB, it may not detect all forms of EPTB, particularly those with low bacillary load, leading to potential underdiagnosis in paucibacillary cases. Additionally, for patients with CBNAAT-negative results, the authors were not able to consistently obtain data on follow-up diagnostic investigations or alternative diagnoses, which limits our ability to fully evaluate diagnostic accuracy in these cases.
Lastly, this study focuses on a single tertiary centre with a high tuberculosis burden, which may limit the generalisability of their findings to settings with differing TB prevalence or diagnostic capabilities. Future studies could benefit from multi-centre data and include various diagnostic tools to enable a comprehensive evaluation of diagnostic pathways for EPTB.







