Abstract
Background: Excessive antimicrobial consumption may saturate the relationship between subsequent antimicrobial exposure and resistance. The ResistAZM randomised controlled trial in Belgium found that 2 g of azithromycin had no effect on the prevalence of macrolide resistance in oral streptococci. At baseline, macrolide resistance was, however, pervasive in this population of men who have sex with men with high exposure to antimicrobials.
Methods: The authors used the same sampling and laboratory protocol to assess if the streptococcal azithromycin susceptibilities in the ResistAZM study were higher than those from isolates obtained from the Belgian general population with lower consumption of antimicrobials (ComCom2023 study).
Results: Streptococcal azithromycin minimum inhibitory concentrations (MIC) were higher in the ResistAZM (median: 32 mg/L; interquartile range: 12–96 mg/L) than in the ComCom2023 study (median: 1 mg/L; (interquartile range: 0.5–12 mg/L; P<0.00001). The azithromycin MICs of S. mitis/oralis, S. parasanguinis, and S. sanguinis were all significantly higher in the ResistAZM study.
Interpretation: The authors found that the streptococcal macrolide MIC distribution of the ResistAZM participants was significantly higher than that of the Belgian general population in 2023. These findings are compatible with the saturation hypothesis and strengthen the argument that more discriminatory methods are needed to evaluate the effects of antimicrobials on antimicrobial resistance in populations exposed to high levels of antimicrobials. The authors’ findings add support to stewardship initiatives that aim to reduce antimicrobial consumption in this population.
Key Points
1. Streptococcal macrolide minimum inhibitory concentrations were high in both the general population and men who had sex with men.
2. Streptococcal macrolide minimum inhibitory concentrations were significantly higher in men who have sex with men than those in the general population.
3. More discriminatory methods are needed to evaluate the effects of antimicrobials on antimicrobial resistance in populations exposed to high levels of antimicrobials.
INTRODUCTION
A recent randomised controlled trial came to the surprising conclusion that dual gonococcal treatment with azithromycin/ceftriaxone did not increase the proportion of commensal Neisseria spp. or streptococci with azithromycin resistance compared to ceftriaxone monotherapy.1 Two reasons for this finding were advanced in the ResistAZM trial. First, measuring the minimum inhibitory concentrations (MIC) distribution of target bacteria is a more sensitive method of detecting reduced susceptibility than the proportion of colonies that are resistant.2-4 Second, the extent of antimicrobial resistance (AMR) seen in this study may have saturated the relationship between antimicrobial consumption and resistance.1 Almost half the participants reported using antimicrobials in the year prior to the study, and hence, their streptococci and Neisseria spp. from their baseline visit displayed high levels of AMR. For example, all the individuals had streptococcal isolates with azithromycin MICs greater than 2 mg/L.
To explore the first explanation, the authors repeated the analyses using single colony MICs and confirmed that the samples from 14 days post-treatment with azithromycin did have higher azithromycin MICs for both streptococci and commensal Neisseria spp. compared to baseline samples.2 In the current manuscript, the authors explore the second hypothesis by comparing the streptococcal azithromycin MIC distributions of the baseline samples of the ResistAZM study with samples from the Belgian general population that were collected using a very similar methodology to that used in the ResistAZM trial.
A second objective was to compare the streptococcal azithromycin MICs from the general population with those from previous surveys. This comparison aimed to determine if there has been a shift in azithromycin MIC over time. The data from previously published surveys on streptococcal macrolide resistance in Belgium are not comparable with the authors’ study. One study in 2001 found that the prevalence of macrolide resistance in viridans group streptococci detected from pharyngeal swabs of 154 Belgian individuals aged 17–25 years was 71%.5 A randomised controlled trial of medical students in Antwerp, Belgium, between 2002–2003 found that approximately 25% of participants had at least one isolate of oral streptococci with erythromycin resistance (erythromycin MIC >2 mg/L) in their baseline samples.6 Individual colony MICs and species identities were not reported in either of these studies. The only comparable data the authors found was from the Antimicrobial Testing Leadership and Surveillance (ATLAS) collection of Streptococcus pneumoniae isolates from Belgian sputum isolates. For this second objective, the authors, therefore, limited their analysis to comparing the azithromycin MICs of S. pneumoniae in the ATLAS collection with those from their general population sample.
METHODS
ComCom2023 Survey Population
The antimicrobial susceptibility of Commensals in the Community 2023 (ComCom2023) study was conducted over the course of two weekends in October 2023. Thirty-five randomly selected families attending children’s sports events at a municipal sporting facility in Antwerp, Belgium, were recruited. The full study methodology has been published elsewhere.7 Briefly, the inclusion criteria required being a member of a family where at least one child (aged 5–13 years) and one adult who was either the parent or a first-degree relative living with the child were willing to participate. The participating child needed to be present with at least one of the parents.
ResistAZM Study Population
The ResistAZM study methodology has been published elsewhere.8 Briefly, this was an randomised controlled trial comparing the effect on the resistome of ceftriaxone 1 g intramuscular injection plus azithromycin (AZM) 2 g orally versus ceftriaxone 1 g intramuscular injection alone for the treatment of N. gonorrhoeae. Twenty men who have sex with men (MSM) with genital, anorectal, or pharyngeal N. gonorrhoeae infection and no consumption of a macrolide in the preceding 6 months were randomised into the ceftriaxone/azithromycin arm, and 22 to the ceftriaxone arm. Secondary outcomes included the proportion of oral streptococci and commensal Neisseria spp. that were resistant to azithromycin taken from oral rinse samples.
Sampling Procedure
The same sampling method and sample processing were used in both studies. For both studies, participants were instructed to rinse/gargle their mouths with 15 mL sterile water for 30 seconds, after which this was collected in a sterile container.9 Immediately upon arrival at the laboratory (within 6 h after sample collection), 750 µL of each sample was added to 750 µL of skim milk with 30% glycerol and stored at -80 °C until further processing in batch.
Sample Processing
Culture, MIC determination, and identification of streptococcal species
One aliquot of each sample in skim milk was thawed at room temperature, vortexed, and 100 µL was spread plated onto Columbia CNA agar plates with 5% sheep blood (Beckton-Dickinson, Belgium). Plates were incubated for up to 48 h at 37 °C in 5–7% CO2 incubator. After 24–48 hours of incubation, a minimum of four colonies with streptococcal morphology were randomly selected. Species identities were confirmed by matrix-assisted laser desorption ionisation-time of flight mass spectrometry (MALDI-TOF MS) as previously described.10 S. mitis and S. oralis were identified as S. mitis/oralis.11 MICs were ascertained via Etests (BioMérieux, France) on Mueller Hinton agar with 5% horse blood and 20 mg/l ß-NAD (BioMérieux, France).
Historical S. pneumoniae azithromycin susceptibility: ATLAS collection
The authors used the ATLAS database to provide a historical comparison of azithromycin MICs in the Belgian general population. ATLAS holds 6 million anonymised MICs for 3,919 pathogen–antibiotic pairs isolated from 630,000 individuals in 88 countries between 2004–2017.12 Comparisons of ATLAS antimicrobial susceptibility results with those from other datasets, such as the European Centre for Disease Prevention and Control (ECDC) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST), have revealed that the isolates in ATLAS have a similar but slightly higher MIC distribution per species-antibiotic pair than the other datasets.12
The ATLAS dataset contains azithromycin MICs for 706 S. pneumoniae isolates from Belgium. Of these, the authors used the 468 that were isolated from sputum isolates.12 These isolates were obtained between 2005–2017 (median 2013; interquartile range [IQR]: 2009–2015).
Statistics
When MICs were reported as less or more than the lowest or highest concentration tested, these were replaced with the lowest or highest concentrations tested, e.g., azithromycin MIC >256 mg/L was recorded as 256 mg/L.
Differences in MICs were compared between groups using Wilcoxon rank sum tests. The Bonferroni correction was applied to account for multiple comparisons. Statistical analyses were conducted using Stata v16.1 (StataCorp, LLC College Station, Texas).
Ethics
Ethics approval was obtained from ITM’s Institutional Review Board (1574/22) and the Ethics Committee of the University of Antwerp (3831).
RESULTS
Survey Population Data
The participants of the ResistAZM were older than those in the Comcom2023 study but similar in age to the adults in the ComCom2023 study (Table 1).
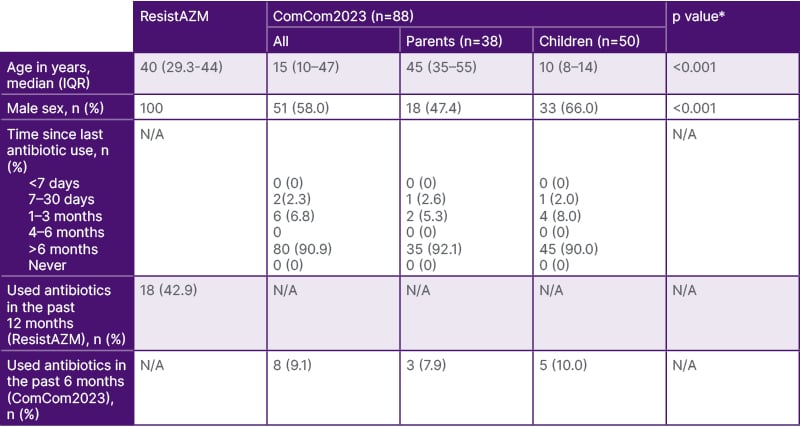
Table 1: Population characteristics.
*Willcoxon rank sum test (age) or Fisher’s exact test (sex)
IQR: interquartile range; N/A: not applicable.
While all the participants in the ResistAZM study were men, only 51% (51/88) of the participants were men in the ComCom2023 study. Eighteen of 42 (42.9%) participants in the ResistAZM study reported using antimicrobials in the 12 months prior to the study, whereas 8/88 (9.1%) of the ComCom2023 study reported antimicrobial use in the prior 6 months. None of the ResistAZM participants had taken macrolides in the prior 6 months. In the 12 months before recruitment, 14 had consumed beta-lactams, seven tetracyclines, one a fluoroquinolone, and one a macrolide.
Streptococcal Species Composition
S. mitits/oralis was the most abundant streptococcal species in the ResistAZM-baseline-visit (34/81 isolates, 42%) and the ComCom2023 (146/312, 46.8%) studies (Figure 1; Supplementary Table 1). S. sanguinis was detected more frequently in the ResistAZM (24.7%) than in the ComCom2023 study (6.1%; P<0.001). In contrast, S. salivarius was detected more frequently in the ComCom2023 (17.9%) than in the ResistAZM study (4.9%; P<0.001).
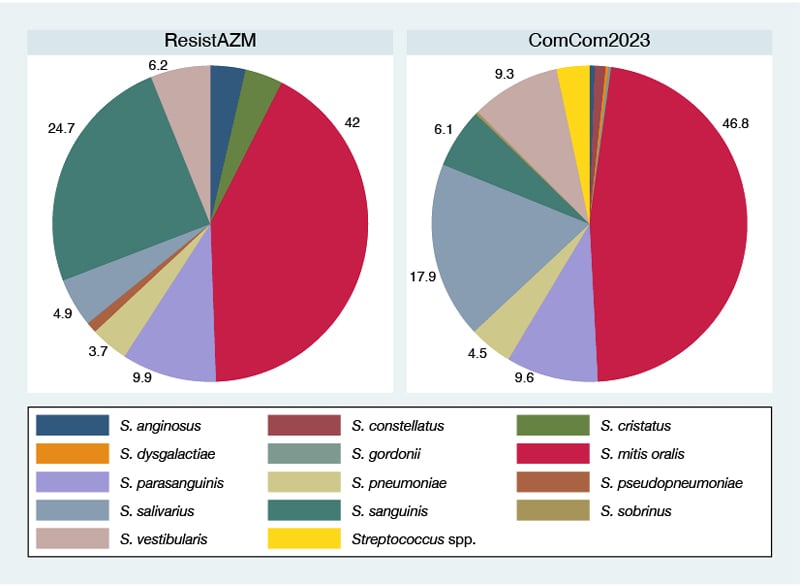
Figure 1: Streptococcal species composition in the ResistAZM and ComCom2023 studies.
The pie charts depict the percent of each streptococcal species isolated out of all streptococcal isolates from that study (only the baseline results are shown for the ResistAZM study).
Antimicrobial Susceptibility of Streptococci
Streptococcal azithromycin MICs were higher in the ResistAZM (median: 32 mg/L; IQR: 12–96 mg/L) than the ComCom2023 study (median: 1 mg/L; IQR: 0.5–12mg/L; P<0.00001; Table 2). The azithromycin MICs of S. mitis/oralis, S. parasanguinis, and S. sanguinis were all significantly higher in the ResistAZM study (P<0.0001; [hlTable 2[/hl]). There was no significant difference between the streptococcal species composition or azithromycin MICs by species between the children and the adults in the ComCom2023 study (Supplementary Table 2).
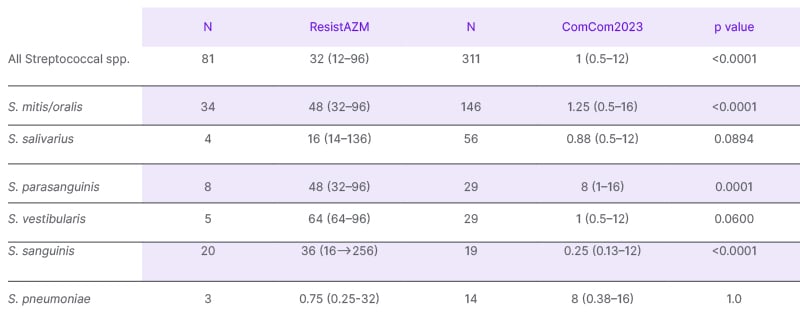
Table 2: Azithromycin MIC (mg/L) in the ComCom2023 and the baseline samples of the ResistAZM study.
MIC: minimum inhibitory concentration; N: number.
Using the EUCAST azithromycin breakpoint of 0.25 mg/L for S. pneumoniae and Group A, B, C, and G streptococci, only one of 81 (1.2%) isolates from the ResistAZM study was susceptible to azithromycin compared to 29/331 (9.3%; P<0.001) in the ComCom2023 study (Supplementary Table 3).13 If a breakpoint of 2 mg/L is used, then 79.1% of ResistAZM isolates and 46.0% of ComCom23 isolates would be classified as resistant (Supplementary Table 3 and Supplementary Table 4).
Switching to considering the prevalence of AMR per person, 78/88 (88.6%) of individuals in the ComCom2023 study had streptococci with MICs >2 mg/L (Supplementary Table 5). The same was true for 100% of the ResistAZM cohort.
ATLAS versus ComCom2023
The median azithromycin MIC of the sputum S. pneumoniae isolates in the ATLAS collection was 0.06mg/L, IQR 0.06-0.25mg/L (Supplementary Table 6). This was significantly lower than the S. pneumoniae MICs from the ComCom2023 study (median 8mg/L; IQR: 0.38-16; P=0.0003).
DISCUSSION
The authors found that the streptococcal azithromycin MICs of the participants in the ResistAZM study were markedly higher than those from the ComCom2023 study. This was driven by higher azithromycin MICs in three of the most prevalent streptococcal species (S. mitis/oralis, S. parasanguinis, and S. sanguinis). Only minor differences in the composition of streptococcal species were found between the studies.
These findings are compatible with the AMR saturation hypothesis. Close to 100% of isolates from the ResistAZM would be classified as resistant to azithromycin according to the EUCAST breakpoint. The median azithromycin MIC was 32 mg/L in this study, with only 20.9% of isolates having a MIC below 2 mg/L. These high MICs would be expected to decrease the chance of detecting an azithromycin-induced increase in the proportion of colonies with azithromycin resistance (defined as >2 mg/L in the ResistAZM study).
It is likely that high consumption of antimicrobials is responsible for the high azithromycin MICs in the ResistAZM population. Almost half of the study population reported consuming antimicrobials in the prior year. The association between antimicrobial consumption and resistance is, however, not straightforward. Consumption of macrolides in the prior 6 months was an exclusion criterion for study entry. Only one individual had thus consumed a macrolide, and this was between 6–12 months prior. The effect of macrolide consumption on oral streptococcal macrolide resistance has been shown to last for 6 months, but the possibility that this effect could last longer cannot be excluded.6 It is also possible that the other antimicrobials could select for resistance to macrolides. However, a multivariate linear regression analysis found no association between consumption of antimicrobials and median streptococcal azithromycin MIC per individual.1,14
Various lines of evidence suggest that population-level consumption of antimicrobials plays a key role. There are at least five different population-level pathways between antimicrobial consumption and resistance.15,16 The authors have established that macrolide consumption in their MSM preexposure prophylaxis cohort was until recently up to seven-fold greater than macrolide consumption thresholds associated with macrolide resistance in bacteria such as M. genitalium and S. pneumoniae.17,18 From detailed analyses of this cohort, the authors have confirmed that oral Neisseria species do have higher macrolide and ciprofloxacin MICs than those from the Belgian general population.10 Surprisingly, these elevated MICs were found in both the MSM who had and who had not consumed antimicrobials in the prior 6 months.10 There was no difference in the MICs between these two groups of MSM.10 Likewise, individual-level analyses found no association between recent antimicrobial consumption and ceftriaxone, azithromycin, or ciprofloxacin MICs.14 These findings, plus the extensive evidence of transmission of resistant bacteria between individuals, have led the authors and others to conclude that population-level consumption of antimicrobials is a key determinant of high MICs in this population.10,19,20
The azithromycin MICs of the general population (ComCom2023) were also concerningly high. The authors found that the pneumococcal azithromycin MICs were higher than those in ATLAS, which were collected between 2005–2017. The authors have very little metadata for the ATLAS collection, and therefore, these differences may simply stem from selection biases. It is likely that ATLAS contains more invasive isolates of pneumococcus, which have been found to have lower beta-lactam MICs than non-invasive isolates.21 Previous comparisons of ATLAS with other datasets have, however, found that ATLAS tends to be slightly biased toward less-susceptible isolates.12
There are a number of other limitations to the authors’ analysis. The authors did not assess the MICs in duplicate. The sample sizes of the studies are small. ResistAZM and ComCom2023 were not conducted in the same season, which may mean that season variations in MIC may contribute to the differences in MIC the authors found.22 Species identification relied on MALDI-TOF MS, which lacks sufficient discrimination to distinguish species such as S. mitis from S. oralis. Finally, the authors did not identify the molecular basis of macrolide resistance. Future studies could circumvent these limitations by conducting similar but simultaneous surveys in general populations and key populations with high antimicrobial consumption using larger sample sizes. Antimicrobial susceptibilities to a broader range of antimicrobials could be assessed. Whole genome sequencing of isolates would enable optimised species identification and comparisons of the prevalence of macrolide-resistance-associated mutations.
CONCLUSION
The authors found that the streptococcal macrolide MIC distribution of the ResistAZM participants was alarmingly high and significantly higher than that of the general population in 2023. These findings are compatible with the saturation hypothesis and add extra weight to the argument that more discriminatory methods than the proportion resistant are needed to evaluate the effects of antimicrobials on AMR in this population.3 Crucially, the authors’ findings add support to stewardship initiatives that aim to reduce antimicrobial consumption in this population. As an example, a single intervention in the authors’ HIV preexposure prophylaxis clinic was able to reduce macrolide consumption by fourfold.23







