Abstract
Obesity is a lifestyle disease that is a proven predisposing factor for many illnesses and is often associated with a poor prognosis. Here, the author tries to associate the relationship between the incidence of obesity in patients with cancer and the prognosis of the same. The present medical literature suggests an ambiguous and conflicting relationship. This study presents an extensive literature review of the mechanisms that may govern the survival outcomes of patients with cancer presenting with obesity. Medical literature databases, namely PubMed, Google Scholar, and BioMed Central databases, were searched. Out of 335 relevant results, 75 met the inclusion criteria. The results were varying in nature, with some papers showing poor prognosis due to the association of obesity with metabolic and endocrine abnormalities, which promote tumour growth, while others suggest that excess adiposity may promote a greater expression of programmed cell death protein-1 in effector CD8+ T lymphocytes, promoting a better response to immune checkpoint blockade therapies. Some even argue against the existence of the so-called ‘obesity paradox’, considering it a by-product of statistical misinterpretation and biases. In conclusion, the phenomenon is definitely intriguing but needs further investigation and research regarding other processes that may all in all affect cancer prognosis.
Key Points
1. Obesity is a known negative prognostic factor for many diseases and is associated with higher risk of all-cause mortality. However, few recent large scale meta-analyses state that some patients with cancer with a high BMI may have better prognosis and survival outcomes than patients with a normal BMI.
2. This review article explores various explanations for the existence of the so-called ‘obesity paradox’ and the arguments given against the existence of the same focusing on the immunological aspects of the observation.
3. Although the positive linkage between obesity and prognosis of specific cancers is statistically significant and has strong mechanistic explanations backed by many studies, the bigger picture is that obesity should still be viewed as an overall negative marker for cancer-specific prognosis. The positive linkage may only be of clinical significance in the treatment response of immune checkpoint inhibitor therapy.
INTRODUCTION
Obesity, defined as a BMI greater than or equal to 30 kg/m2, is a condition with rising incidence around the world that contributes to other health issues.1 Body fat is a negative prognosis factor for many illnesses. Large-scale studies have evaluated the relationship between obesity and mortality. A meta-analysis of more than 200 studies found that obesity was associated with a higher risk of overall mortality.2 An increased BMI is associated with an increased risk of various types of malignancies.3
Furthermore, obesity may increase cancer specific mortality.4 The pathophysiological mechanisms that contribute to higher cancer incidence may include variations in sex corticoid metabolism, insulin levels, and adipokine pathways.1 However, some studies state that patients with cancer with a high BMI (≥30 kg/m2) may have better prognosis and survival outcomes than patients with a normal BMI (20–25 kg/m2). This finding, known as the ‘obesity paradox’, pre-exists in the cardiac literature but less so in oncology.5 The recurrent observation of the ‘obesity paradox’ has led to an increasing amount of research being conducted to find a suitable explanation. Explanations range from observations that contradict causality owing to confounding, to clinical explanations, such as seeking explanations for obesity being protective in varied circumstances.6
However, one particular clinical explanation has gained significant attention from the scientific community, namely the association of obesity with increased efficiency of programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) checkpoint blockade.6 This greatly increases the T cell response to cancer. Checkpoint blockers are becoming a highly promising therapeutic approach yielding remarkable antitumour responses.7 However, despite the success of PD-(L)1 blockade in various malignancies, it fails to generate sustained beneficial outcome in the majority of patients.6 In this review, the author tries to demonstrate the marked effects of obesity on different types and forms of cancer, with particular emphasis on therapeutic outcomes in patients with cancer treated with PD-L1 checkpoint blockade categorised by body mass. This review highlights expected as well as contrasting effects of obesity on cancer prognosis, primarily focusing on, but not limited to, immunotherapy.
MATERIALS AND METHODS
This paper provides an overview of the various proposed mechanisms by which obesity can have varying effects on cancer prognosis. An elaborate literature search was performed in the PubMed, Google Scholar, and BioMed Central databases. The keywords used for the search were “obesity paradox,” “immune checkpoint inhibitors,” “PD-1/PD-L1 blockade,” “BMI,” “obesity,” and various combinations of these words. A total of 75 articles were reviewed.
The publications were selected as per the following inclusion criteria: the objective of the research should be related to the effects of obesity on cancer prognosis; the literature showcasing immunotherapeutic mechanisms was preferred; and papers showing contrary results by the same mechanisms and methods were given due diligence. Exclusion criteria primarily included research papers in which the effect of obesity was not a specific aim or other comorbidities were present in the participants.
RESULTS
The author’s literature search yielded 335 articles, of which 75 (22.3%) met the inclusion criteria for the overall literature review of obesity and its link with cancer outcomes with regard to overall survival, cancer-specific survival, and progression-free survival.
Obesity and Cancer: The Link
Evidence for increased risk of cancers
Clinical evidence establishes the proof of a significant association, backed by data, between obesity and various cancers.8 The pooled risk ratios of a particular study are shown in Table 1.
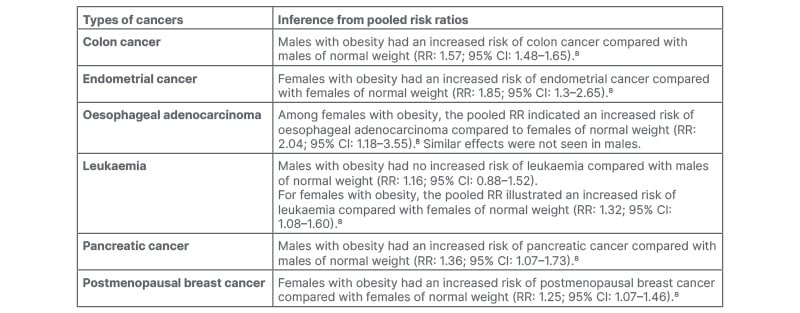
Table 1: Link between obesity and various types of cancers.
CI: confidence interval; RR: risk ratio.
A particular reason can be that obesity is linked with endocrine abnormalities, such as changes in sex corticoid metabolism, insulin levels, and inflammatory pathways, all of which have been shown to stimulate tumour growth.9 Adipokines also contribute to Type 2 diabetes, causing higher insulin and insulin-like growth factor-1 levels, which in turn promote tumour growth.10 Sex corticoid metabolism and chronic inflammation mediate the relationship between obesity and cancer, while the evidence for a role of insulin and insulin-like growth factor signalling is, as such, moderate.9 Aromatase, which converts androgens to oestrogens, is found in adipose tissue and is increased in obesity, thus increasing blood oestrogen levels.11 7β- oestradiol can induce neoplastic growth in human breast, as proven by the proliferation of neoplastic cells in mice with severe combined immunodeficiency disease.12
Additionally, there is strong conviction that weight loss positively affects the mechanisms mentioned above. Data on body weight loss have suggested that intentional weight loss, including surgical procedures, may reduce cancer risk.13,14 However, a particular contrasting study states that while bariatric surgery does reduce cancer risk in females, no such effect was seen in males undergoing bariatric surgery.10 Lifestyle change to affect intentional weight loss in patients with obesity normalises circulating cancer risk factors, including oestrogens, Insulin-like growth factor 1, and inflammatory cytokines.10 Additionally, the use of pharmacological approaches to tackle the problem of obesity has not resulted in obesity control at either individual or population levels.15 Considering animal models, collective data have shown that obesity in rodents promotes tumourigenesis and increases the incidence of many types of cancer.16
Another study reported observations showing that high-fat diets and obesity cause colon epithelial cells in mouse models to remodel their enhancer landscape analogously to the remodelling of the enhancer landscape seen in colorectal cancer.17
The obesity paradox: does it exist?
Clinical research pursued in recent years has reported lower mortality in subjects who are overweight (BMI of 25–30 kg/m2) affected by different types of cancer,18,19 although statistical significance varies and is questionable. In a particular meta-analysis, risk reduction of death by 3.6% was seen for every 1 kg/m2 increase in BMI.20 Obesity was found to be linked with higher efficacy of PD-1/PD-L1 blockade in both tumour-bearing mice and clinical patients with cancer.6 Additionally, the immunosuppressive microenvironment induced by obesity can make immune checkpoint inhibitors more sensitive.21 Although the literature also states that obesity is an increased risk factor for other types of cancer, a particularly interesting case is the curious scenario of the relation of obesity with colorectal cancer. With conflicting results observed in different studies, one particular meta-analysis, which investigated the relationship between post-diagnostic BMI and colorectal cancer, concluded that there was lower mortality among patients with obesity.22
Another meta-analysis of 29 studies concluded that there is increased mortality in patients with obesity and colorectal cancer. It further stated that significant heterogeneity is observed, which is attributed to the timing of BMI assessment.23 The link between obesity and a worse prognosis was cemented when BMI was calculated before diagnosis. This particular observation is strengthened by other literature that claims the ‘obesity paradox’ is not a paradox at all; rather, the studies in favour of paradoxical effects of obesity represent misclassification bias caused by the use of BMI as a measure of obesity, reverse causation, or collider stratification bias.24 A particular study argues that the obesity paradox is caused by the presence of statistical biases. Although these biases are present in most studies, they are exaggerated when populations affected by a disease state are considered.25 In the upcoming section, the author will explain the abovementioned biases with respect to the paradox (Figure 1).

Figure 1: Statistical biases causing the formation of the so-called ‘obesity paradox’.
I) Misclassification bias
BMI, as a measure of adiposity, is controversial. It is, by definition, an established fact that BMI can barely differentiate the ratio of fat to lean mass (LM).26 In any scenario, as the percentage of fat rose, so did BMI. On this basis, BMI seems sufficient at measuring adiposity, but there are other factors as well. As an example, BMI is not independent of height during infancy and puberty. Additionally, during these periods, body weight is proportional to the third power of height. Another instance is that, both in the normal weight range and among those with obesity, individuals can have different body compositions even if they have similar BMI.6
II) Reverse causation
It refers either to a cause-and-effect relationship to a common pre-disposing factor or to a two-way causal relationship with each other. This term explains the confounding by weight loss that in turn elevates mortality risk.27 This means that since causative agents of low body weight cause increased mortality risk, it may seem as if high body weight lowers relative mortality, which is nothing but a statistical misinterpretation.
III) Collider stratification bias
This refers to the change in the relationship between two variables when viewed in the narrow context of a disease state. It is also known as ‘event index bias’. The relations between two different exposures (such as alcoholism and obesity) and their relation to mortality are dependent on the effect of being affected by another disease, such as diabetes.6
This effect can change the relationship between the two exposures relative to their relationship in the source population. The effect of this bias caused due to a pre-existing disease state is unpredictable without further information. In different cases, the usual correlation can be reversed or exaggerated due to the pre-existing condition. When reversed, it is referred to as reverse causation bias, as stated above.
As an example, the relation of smoking and obesity among those having diabetes as a comorbidity strengthens the negative association between smoking and obesity.25 The increased incidence of smoking among patients with diabetes will increase the incidence of weight loss relative to the source population.25 Due to the above processes, the lesser mortality of persons who are not obese in the source population can be muted or even reversed in the subpopulation consisting of people with diabetes.25 Among people with diabetes who never smoked, the relationship of higher mortality among those with obesity is sustained. Among non-smokers, there is no collision between obesity and smoking, thus the sources of bias associated with smoking are removed.25
Ambiguous Effects of Obesity on Programmed Cell Death 1 Checkpoint Blockade and Functioning of T Cells
First, T cell exhaustion is higher in patients with obesity across various different species.6 Mice with obesity show an increase in exhausted T cells in the blood, spleen, and liver. They also show a higher number of PD-1 positive T cells in the liver, which causes decreased cytokine production and cell proliferation. Similar results were observed in humans and non-human primates. Second, obesity promotes tumour growth.6 T cells originating from mice with obesity having malignant transformations present a transcriptomic profile that is varied from that of T cells from tumour-bearing control mice, findings which suggest increased exhaustion of T cells.6
Third, leptin levels are linked with PD-1 expression.6 The results indicated a role for leptin signalling in increasing PD-1 in tumour-bearing mice, which affects antitumour immunity.6 Signal transducer and activator of transcription 3, a downstream transcription factor of the leptin receptor, has known binding sites in the promoter region of PD-1 and has been implicated in driving expression of PD-1 in human and murine cancers, causing increased expression and better treatment response.6 Fourth, efficacy of PD-1/PD-L1 checkpoint blockade is improved in mice with obesity.6 The excess adiposity may promote increased expression of PD-1 in CD8+ T cells, causing a better response to immune checkpoint blockade therapy.28 Human patients treated with PD-L1 checkpoint blockade showed a significant improvement in progression-free survival and overall survival in patients with obesity. This finding can be explained as follows: in obesity, PD-1 mediated immune suppression may be a naturally evolved mechanism to protect against possible autoreactive T cell responses induced by chronic inflammation.6 So, this increased expression of PD-1 receptor in patients with obesity may account for the higher efficacy of PD-1/PD-L1 checkpoint blockade therapy used for various cancers.
Finally, a new study concluded that patients with obesity are also linked with a higher possibility of experiencing immune-related adverse effects to various degrees, including but not limited to endocrine, gastrointestinal, and liver adverse events, among others, especially rheumatic and pulmonary events.29
DISCUSSION
Understanding the ‘Obesity Paradox’
A BMI of 22.5 kg/m2 is accepted as a mid-reference point for normal weight.30 The so-called ‘obesity paradox’ occurs when the risk of mortality is decreased for BMI values above this value, when an increased risk is expected. When the BMI value is very high, risk either returns to unity or increases.5 Here, BMI fails to contrast whether the excess weight is the result of increased fat mass or LM; thus, it is suggested that the ‘obesity paradox’ in the context of cardiovascular diseases is based on the inability of BMI to differentiate between LM and fat mass. A particular study conducted in India concluded that total LM and regional LM were higher in the obese category than in the normal weight category.31 Research suggests that the positive impact of increased BMI results from the positive effects of lean tissue, which overcomes the negative effects of excess body fat.32
Immunological basis of the obesity paradox: a plausible explanation
To help readers understand the immunological basis of the obesity paradox, the immunological basis of cancer will be explained (Figure 2).
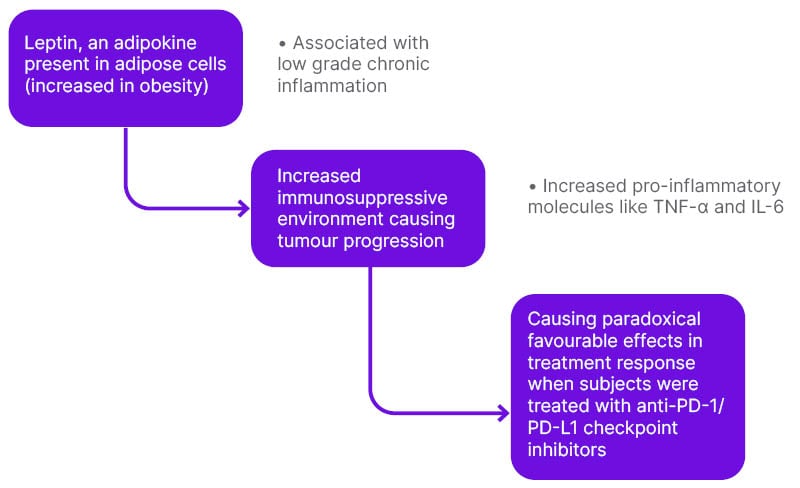
Figure 2: Mechanisms associated with the ‘paradox’ observed causing better treatment response with regard to immune checkpoint blockade therapy.
PD-L1: programmed cell death ligand 1; PD-1: programmed cell death 1.
Immune checkpoint inhibitors
These have emerged as a first step treatment for various malignancies.33 The immune system is instrumental in the management of tumours, as well as in the techniques employed by tumour cells to escape the policing of immune cells, leading to the acceptance of immunotherapy as a new but encouraging approach to tackle this complex interaction between cancer and immunity.33 To protect the body against various pathogens, the immune system has developed various mechanisms and pathways over the course of biological evolution. However, it must do so while recognising and sparing the body’s own cells. This task is accomplished by the presence of multiple checkpoint pathways that regulate immune responses.
Among other negative regulatory receptors that mediate these inhibitory feedbacks, PD-1 has been one of the most intensively studied regulators due to its role in maintaining T cell function and immune system homeostasis. It is a chemokine receptor and is expressed on the T cell membrane. It recognises the body’s own cells by binding to the PD-L1 ligand expressed on those cells. Tumour cells take its advantage to evade T-cells by expressing PD-L1 ligand, which binds to the PD-1 receptor on cytotoxic T-cells, thus rendering the immune response ineffective.33 Thus, a mechanism was proposed, which was essentially the blockade of this checkpoint, and anti PD-1 checkpoint blockers were conceptualised to induce antitumour immune responses.33
PD-1/PD-L1 checkpoint blockade therapy has become one of the mainstream therapies for various malignancies. However, some patients do not show complete remission, and adverse events have been seen, which suggests that a better understanding of immunosuppression mediated by this pathway is needed to predict prognosis and improve treatment.34
Examples include nivolumab and pembrolizumab. Another study showed that patients with melanoma and a high BMI treated with ipilimumab had a greater response.28
CONCLUSION
In the present review, the author showed different perspectives of academia on the existence of obesity, and logical explanations and deductions following them. Furthermore, emphasis was placed on the most weighted counterargument against the paradox that it is a product of statistical biases, namely, misclassification bias, reverse causation bias, and collider bias. Additionally, different explanations were cited, and particular focus was placed on the immunological aspect of the obesity paradox, that is, its ambiguous effect on the PD-1/PD-L1 immune blockade mechanism, mainly consisting of increased T cell exhaustion but increased efficacy of the blockade system. Drugs such as ipilimumab, pembrolizumab, nivolumab, and atezolizumab have been used in cancer immunotherapy with considerable success.
The phenomenon is definitely intriguing but needs further investigation and characterisation regarding other processes that may all in all affect cancer prognosis.







