Abstract
Background: Tuberculosis (TB) has remained one of the top causes of morbidity and mortality in the Philippines since 2018. However, adverse drug reactions (ADR) from first-line anti-tuberculosis medications such as rifampicin, isoniazid, pyrazinamide, and ethambutol cause significant impact on treatment adherence. This study aimed to determine the incidence of ADRs among patients treated with first-line anti-tuberculosis medications at a Philippine Department of Health tertiary medical centre.
Methods: In this retrospective cohort study, rifampicin-susceptible patients undergoing first-line anti-TB treatment enrolled in the Tuberculosis-Directly Observed Treatment Strategy at the East Avenue Medical Centre, Quezon City, Philippines, were included.Pertinent data obtained from chart review included sociodemographic factors, clinical characteristics, duration of treatment prior to the appearance of adverse drug reactions, type of ADRs, TB medication causing the ADR, and treatment outcomes.
Results: A total of 524 patients treated with first-line anti-TB treatment were identified, with 46 (8.78%) noted to have ADRs to the treatment regimen. Most reaction types included mild or localised skin reactions (34.8%), severe skin rash secondary to hypersensitivity (32.6%), and jaundice due to hepatitis (21.7%). Reaction to more than one medication was seen in 89.1% of patients with ADRs, with rifampicin having the highest frequency of ADR (4.3%) among the anti-TB medications.
Conclusion: The incidence of ADRs among patients treated with first-line anti-TB medications at East Avenue Medical Centre in the Tuberculosis-Directly Observed Treatment Strategy is frequent and comparable to other relevant study populations. A larger sample size and exploration of other methodological studies are recommended to further expand on this study.
Key Points
1. According to the WHO, the Philippines ranks fourth among 30 high tuberculosis (TB) burdened countries. Tuberculosis management warrants a prolonged multidrug regimen, thus raising concerns about the impact of adverse drug reactions (ADR) on treatment adherence.2. This is a retrospective cohort study comprising pertinent data obtained from chart review of rifampicin-susceptible patients enrolled in a tertiary medical centre’s TB Directly Observed Treatment Strategy, dating 2021 and earlier.
3. The prevalence of ADRs among patients undergoing first-line TB treatment is frequent. Active surveillance and timely management of these ADRs must be enforced to reduce their impact on treatment adherence.
INTRODUCTION
Tuberculosis (TB) is caused by the bacteria Mycobacterium tuberculosis, a complex, rod-shaped, non-spore-forming, thin, aerobic bacterium.1 Since 2016, it has caused 6.3 million new cases of TB, 95% of which come from developing countries. One of these is the Philippines.2 Treatment is based on different treatment categories for specific indications, namely for Category I treating Pulmonary TB (PTB), Miliary TB, and Extrapulmonary TB (EPTB), except for central nervous system (CNS), bone, or joint infections; Category Ia treating for CNS, bone, and joint infections; Category II for retreatment of rifampicin (RIF) susceptible PTB and EPTB; and Category IIa for retreatment of RIF susceptible CNS, bones, or joint infections.
The virulence of the tubercle bacilli brought about by its properties of resistance to oxidative stress, intracellular survival in macrophages, and high lipid concentration of its cell wall, proved the need for a prolonged multidrug chemotherapy treatment by a four different drug regimen via RIF, isoniazid (INH), ethambutol (EMB), and pyrazinamide (PZA) for TB. To increase treatment adherence, the WHO has established a treatment control strategy via the Directly Observed Treatment Strategy (DOTS), which employs the commitment of the government to eradicate the disease. Processes such as case detection via sputum smear microscopy, standardised treatment regimen directly of 6–9 months, provision of the drug, and standardised recording and reporting system that allows assessment of treatment results.2 However, these drugs are known to have caused adverse events.3 Their management poses challenges as they may cause clinicians to shift drugs to second-line treatment options, lead to undertreatment, or impact adherence to medication.4 There are no published data on the prevalence of adverse reactions in the country to date.
This study aimed to determine the incidence, characteristics, significant risk factors, and outcomes of adverse drug reactions (ADR) among patients treated with first-line anti-tuberculosis medications at a Department of Health tertiary medical centre. Significant differences between treatment outcomes of patients with ADRs to a single first-line anti-tuberculosis medication compared to patients with ADRs to more than one first-line anti-tuberculosis medication were also determined. Established data in this study surrounding adverse reactions to anti-TB medications can be beneficial economically to allocate resources in preventing these adverse reactions with regards to optimising the drugs’ pharmacokinetics and pharmacodynamics.
METHODOLOGY
Definition of Terms
ADRs were defined according to the listed ADRs in Table 12 of the DOH National Tuberculosis Program Manual of Procedures, 6th edition.2 Minor ADRs include gastrointestinal tolerance, mild or localised skin reactions, orange-coloured urine, burning sensation in the feet due to peripheral neuropathy, arthralgia due to hyperuricemia, and flu-like symptoms (fever, muscle pains, inflammation of the respiratory tract). Major ADRs include severe skin rash due to hypersensitivity, jaundice due to hepatitis, impairment of visual acuity and colour vision due to optic neuritis, oliguria, or albuminuria due to renal disorder, psychosis, convulsion, thrombocytopenia, anaemia, and shock.
Research Design
The study employed a retrospective cohort study. The investigators gathered data from the East Avenue Medical Centre TB-DOTS department from years earlier than 2021 through chart review until the study population size was met. Data gathering utilised an online data collection form made especially for the study. Only pertinent data for the sociodemographic (sex, age), clinical characteristics (TB case type, TB diagnosis type, TB treatment history, comorbidities), duration of treatment prior to the appearance of ADRs, TB medication causing the ADR, and treatment outcomes were obtained from the charts for review. The diagnosis of ADRs were obtained from the examining physician’s assessment in the reviewed chart. Symptoms such as mild gastrointestinal disturbances, hepatotoxicity, ototoxicity, nephrotoxicity, peripheral neuropathy, and cutaneous ADRs were determined. Patients were selected based on the inclusion and exclusion criteria (Table 1).

Table 1: Inclusion and exclusion criteria.
Statistical Analysis
SPSS version 27.0 was used for data processing and analysis. Mann-Whitney U test was used to compare non-normal continuous variables, while Chi-square test was used to compare categorical non-continuous variables and, in cases where this was not applicable, Fisher’s exact test was used. To determine the factors associated with the presence of ADR, a simple logistic regression was used. P≤0.05 were considered statistically significant.
RESULTS
A total of 524 patients treated with first-line anti-tuberculosis medications were identified (Table 2). From these patients, 46 (8.78%) were identified to have ADRs to the treatment regimen. Sex (M/F) ratio for patients with ADR and without ADR were at 1.875 and 1.795, respectively. Patient age ranges were from 19–85 years, with median age for patients with ADR at 42.5 years and for patients without ADR at 45 years. The majority of cases presented with pulmonary TB, clinically diagnosed TB, and as new treatment cases. More ADR was seen in patients with diabetes as compared to patients with HIV.
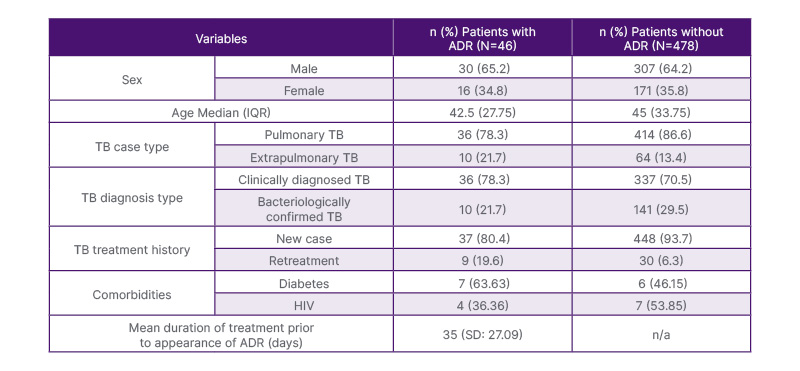
Table 2: Sociodemographic and clinical characteristics of patients with and without adverse drug reactions to first-line anti-tuberculosis medications in East Avenue Medical Centre tuberculosis Directly Observed Treatment Strategy, from earlier than 2021.
Note: Other comorbidities not specified were not included.
IQR: interquartile range; TB: tuberculosis.
The top three reaction types in decreasing order of frequency (Table 3) included mild or localised skin reactions (34.8%), severe skin rash secondary to hypersensitivity (32.6%), and jaundice due to hepatitis (21.7%). Reaction to more than one medication was seen in 89.1% of patients with ADRs. Among the anti-TB medications, rifampicin has the highest frequency of ADR (4.3%). While the majority of outcomes of patients with ADR were classified as treatment completed, the outcome was not statistically significant.
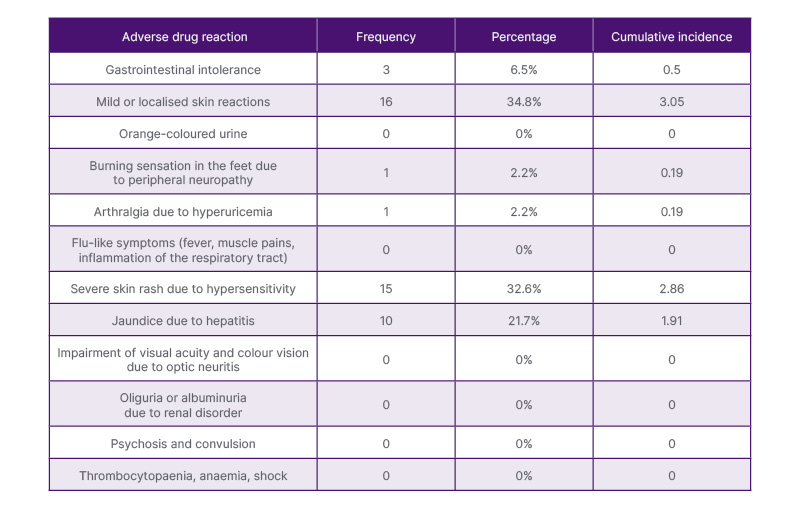
Table 3: Frequency distribution and incidence of adverse drug reaction types among patients with adverse drug reactions to first-line anti-tuberculosis medications.
Using simple logistic regression analysis to determine significantly associated variables to the presence of ADR, patients with extrapulmonary TB have about 2.231 higher odds of having ADR than those that have pulmonary TB. Patients under retreatment have about 0.280 lower odds of having ADR than those treated as new cases. The TB history of a patient was also associated with the presence of ADR and vice-versa.
DISCUSSION
Sociodemographic (sex, age), clinical characteristics (TB case type, TB diagnosis type, TB treatment history, comorbidities), duration of treatment prior to the appearance of ADRs, TB medication causing the ADR, and treatment outcomes were obtained. In this study, among a total of 524 patients on first-line anti-TB medications, 8.78% of patients were noted to have ADRs. The median age for those with ADRs was 42.5 years old, which is younger than the cited age (>60 years old) at risk for developing ADRs.
Of the common adverse drug reactions that were cited, multiple studies have shown that the most frequently observed ADRs included gastrointestinal and hepatobiliary manifestations.5-14 In particular, nausea, vomiting, and epigastric pain were the more commonly cited gastrointestinal manifestations.15 On the contrary, in this study, the most common ADR was found to be skin manifestations, ranging from mild or localised skin reactions (34.8%) to severe skin rash secondary to hypersensitivity (32.6%), followed by jaundice due to hepatitis (21.7%). However, there is a paucity in available related literature detailing why skin manifestations were found to be the most frequent ADR.16
In patients having ADR with anti-TB medications, approximately 20% have symptoms of tingling and burning sensation in the hands and feet. This was most attributed to treatment with isoniazid and, to a lesser degree, with ethambutol.17 In a study conducted in Shenzhen, China, optical neuritis also occurred concomitantly with peripheral neuritis, but this was associated with when linezolid was used as treatment for MDR-TB.18 The study employed the Michigan Neuropathy Screening Instrument (MNSI) and serum trough levels for monitoring of linezolid neurotoxicity. This study reported a single patient having self-reported symptoms of a tingling sensation. Data was lacking as to which drug was attributable for the event as this was reported upon intake of a fixed-dose combination drug.
One notable significant finding in this study is that patients with extrapulmonary TB have about 2.231 higher odds of having ADR than those who have pulmonary TB. This result, however, was not consistently reflected in other studies that showed varying results from the predominance of ADRs in pulmonary TB19, predominance of ADRs in extrapulmonary TB20-22, and no significant difference between the two groups.23
Patients under retreatment have about 0.280 lower odds of having ADR than those treated as new cases. The TB history of a patient was also associated with the presence of ADR and vice-versa.
Among those infected with TB, HIV and diabetes were of particular interest due to their strong association with the disease.24 A state of immune deficiency is observed in these comorbidities due to their defects in bacterial recognition, phagocytic activity, and cellular activation which results in impaired production of chemokines and cytokines to prevent TB infection.25 In patients with diabetes or HIV, oxidative stress and polypharmacy are believed to increase the risk for ADRs.16, 26
Comparing the present study population proportion to known population proportion from literature, there appears to be a significant difference between it and that of a multiregional study in China in 2013.17 Multicentre studies in Iran in 20147 and New Delhi, India in 202027, and single hospital studies in Kerala, India in 201513, Ghana in 202128, and Pune, India in 20215 were also noted to have significant differences with the study population.
The current study’s mean duration of treatment prior to the appearance of ADR is 35 days, with a standard deviation of 27.09 and the duration ranging from 6–90 days. This is found to be statistically longer compared to a single hospital study in Kerala, India in 201513, a multicentre study in Iran in 20147, and a 6-year prospective study in Brazil in 2016.29
In this study, the outcomes of patients who developed ADRs were mostly treatment completed, although the outcome was not statistically significant. Comparing these results to previous studies, there was a noted dissimilarity, as ADRs could be associated both with unfavourable8 and favourable3 outcomes.
CONCLUSION
The study showed the incidence of ADRs, the outcomes, determination of known risk factors, and mean duration of patients taking anti-TB medications enrolled at the East Avenue Medical Centre TB-DOTS clinic as discussed.
Very sparse literature is available in the Philippines that detail the incidence of ADRs among those who are receiving anti-TB treatment and the other factors that may influence it. This study’s data demonstrated a few key differences between the international and local settings, such as the younger median age at risk of developing ADRs (42.5 years old) and which ADRs are more common, notably the skin manifestations as compared to the gastrointestinal and hepatobiliary manifestations.
There are notable limitations to this study. The applicability of the inferences made from the results may be dampened by the small sample size and that it was only made from data collated from one local tertiary hospital. A larger sample size and employment of other methodological studies are warranted to further expound on this study.
A successful course of TB treatment relies largely on the patient’s compliance with the medications. Given the multidrug constitution of the anti-TB regimen, the concomitant comorbidities such as diabetes and HIV, and the overall length of duration of the regimen, the appearance of one or more ADRs may be unavoidable and poses a significant threat to the adherence to anti-TB medications. Awareness and immediate recognition of these ADRs will contribute to better adherence, more bolstered safety monitoring, and aid in promoting the rational use of medicines.
This study also suggested the possible variation of risk factors for ADRs across regions. Although, as mentioned, a larger sample size from a multi-centre study can further establish this.
Exploration of other disease manifestations of tuberculosis, notably on extrapulmonary symptoms, and their association with ADRs is also recommended and warrants further exploration. The paucity of available relevant literature exploring the rationale of higher incidence of ADRs in extrapulmonary TB serves also as a notable limitation and an area for further studies.
Given the strong link between the known risk factor of diabetes in developing ADRs, a longitudinal study may be better suited to further elicit this response.
In the era of globalisation and constant migration of people wherein the threat of disease spread is ever-growing, it is important to tackle possible obstacles such as ADRs to effective treatment of communicable diseases such as tuberculosis to prevent its spread on a global scale.
ETHICAL CONSIDERATIONS
A waiver of consent approved by the East Avenue Medical Centre Institutional Ethics Review Board was obtained for the retrospective chart review. Obtained data from the data collection form were stored electronically in a Google Drive (Google, Mountain View, California, USA) subjected to the existing privacy policies of Google in compliance to the Philippine National Guidelines for Health and Health-Related Research (2017) and Philippine Data Privacy Act of 2012.







