Abstract
There is a chaotic scenario that exists in the field of prostate cancer (PCa) screening. To balance goals, such as decreasing mortality, avoiding unnecessary procedures, and decreasing the cost of medical care, the pendulum seems to have swung to the side of more restricted screening. The decrease in PCa screening has led to a slowly creeping decline in the favourable outcomes that existed among patients with PCa. If a potential patient or a family member is trying to get clear guidance about PCa screening by searching the internet, they will end up confused by several recommendations from many organisations. It is even more challenging to obtain any clarity about PCa screening for special populations, such as those with a family history of PCa, those of African descent/African Americans, and the elderly. The advent of genomic medicine and precision medicine is an opportunity to identify those at a very high risk of developing aggressive PCa, so that PCa screening can be more actively undertaken among them. In this paper, the authors review the current recommendations by different entities and summarise emerging molecular markers that may help bring clarity to PCa screening. The authors predict that concrete, consensual guidelines will emerge in less than one decade. Meanwhile, this article suggests intermediary steps that will help save lives from PCa mortality, especially for under-represented populations. This paper is a catalyst to stimulate further discussion and serves as a guide to noncancer-specialists for the near future as precision medicine progresses to better understand risk–benefit and cost–benefit ratios in PCa screening.
INTRODUCTION
Prostate cancer (PCa) was the most commonly diagnosed malignancy in 105 countries in 2018 and the fifth leading cause of cancer deaths in males.1 In general, approximately one in nine males will be diagnosed with PCa during their lifetime.2 Despite the high incidence of PCa in the USA and worldwide, PCa is a very indolent disease. In the USA, an average of 2.44% of males will die from PCa.3 In a study by Johansson et al.,4223 untreated patients with PCa were followed for >30 years and showed 41% local progression, 18% progression to distant metastasis, and a mortality rate of 18%. The mean time to development of metastasis was 9.2 years and the mean time to death was 9.5 years.4 The outcome of PCa is highly dependent on the grade classification at diagnosis. A study by Albertsen et al.,5 which followed a cohort of 767 untreated patients with PCa for 15 years, found a 4–11% mortality rate for Gleason Score 2–5, 18–30% for Gleason Score 6, 42–70% for Gleason Score 7, and 60–87% for Gleason Score 8–10.5
The introduction of prostate-specific antigen (PSA)-based screening had a large impact on PCa. Following the introduction of PSA screening, the mean age at diagnosis of PCa decreased.6 The incidence of males with nonorgan-confined PCa decreased from 79.3% in 1984 to 24.7% in 2005.7 Most significantly, the 5-year survival rates for all races improved from 68% in 1975–1977 to 100% for the years 2003–2009.8 Although PSA screening appeared highly beneficial, these results were all retrospective and raised the question of whether PSA screening was actually improving survival or simply detecting earlier and possibly insignificant PCa and in doing so, causing potential harm. Results of prospective studies led to the first recommendations regarding PSA-based PCa screening, which had a large and controversial impact on PCa. While the potential benefit of PCa screening is evident, the question of who and when to screen remains. The authors hypothesised that the concept of individualised medicine can be applied to PCa screening to optimise its benefit to males most at risk.
METHODOLOGY FOR ARTICLE SEARCH CRITERIA
To identify the available and recommended PCa screening tests, the authors completed a PubMed search using keywords: “prostate cancer”, “prostate cancer biomarkers”, “prostate cancer and race”, “MRI in prostate cancer”, and “prostate cancer screening guidelines”. Only the studies
reported in the English language were included. The authors selected the studies published within the past 10 years, as well as reported the secondary sources from reference lists of retrieved articles online.
2012 U.S. PREVENTIVE SERVICES TASK FORCE SCREENING GUIDELINES AND THEIR IMPACT
Table 1 summarises various agencies and their associated PCa screening guidelines. In 2012, the U.S. Preventative Services Task Force (USPSTF) released a recommendation against the use of PSA-based screening for PCa in all males (Grade D).9 Previously, the USPSTF had recommended against PCa screening in males over the age of 75 years (Grade D), but had found data insufficient to assess males younger than 75 years old (Grade I).10 The 2012 recommendation was largely based on the results of two trials: the U.S. Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial and the European Randomized Study of Screening for Prostate Cancer (ERSPC).9
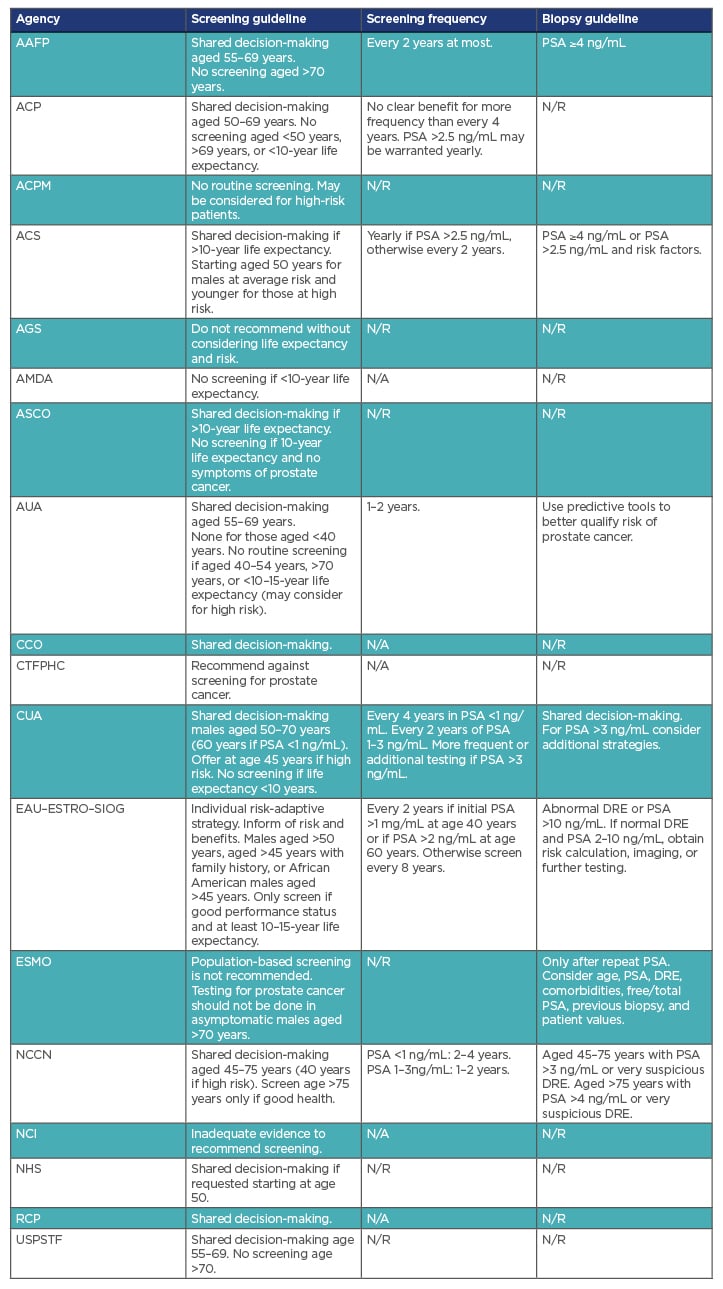
Table 1: Selected international prostate cancer screening guidelines.
AAFP: American Academy of Family Physicians; ACP: American College of Physicians; ACPM: American College of Preventative Medicine; ACS: American Cancer Society; AGS: American Geriatrics Society; AMDA: The Society for Post-Acute and Long-Term Care Medicine; ASCO: American Society of Clinical Oncology; AUA: American Urological Association; CCO: Cancer Care Ontario; CTFPHC: Canadian Task Force on Preventative Health Care; CUA: Canadian Urological Association; DRE: digital rectal examination EAU: European Association of Urology; ESMO: European Society for Medical Oncology; ESTRO: European Society for Radiotherapy and Oncology; N/A: guideline not available; N/R: not recommended; NCCN: National Comprehensive Cancer Network; NCI: National Cancer Institute; NHS: National Health Service; PSA: Prostate Specific Antigen; RCP: Royal College of Pathologists; SIOG: International Society of Geriatric Oncology; USPSTF: United States Preventative Services Task Force.
The PLCO trial was conducted at 10 centres in the USA.11 The study randomised 76,693 males aged 55–74 years to annual screening or usual care. Initial results of a 7–10-year follow-up showed no difference in PCa mortality between the two groups (rate ratio: 1.13; 95% confidence interval [CI]: 0.75–1.7). Follow-up at 13 years also failed to show any significant difference in mortality rates (rate ratio: 1.09; 95% CI: 0.87–1.36).12
The ERSPC was a multicentre, population-based randomised screening trial conducted in eight European countries.13 A total of 162,389 males aged 55–69 years were randomised to a group that offered PSA screening every 4 years or a control group that did not offer screening. At the time of the 2012 USPSTF recommendations, the ERSPC showed a significant reduction in the risk of PCa mortality (approximately 20%); however, nearly 1,000 males needed to be screened to prevent one death.
Based on the results of the PLCO and ERSPC studies, the USPSTF concluded that the potential benefits of PSA-based screening were “at best, very small.”9 The USPSTF also evaluated the risks and harms associated with screening. They considered the high rate of false-positive results, complications of biopsy, and side effects of treatment. These negative consequences of screening were compounded given the perceived high rate of overdiagnosis because of the often-indolent nature of PCa. Thus, the USPSTF concluded with moderate certainty that the benefits of PCa screening did not outweigh the harm, leading to the 2012 recommendations, as mentioned above, against PSA-based screening. The recommendation led to much controversy and numerous studies evaluating the impact on PCa.
The first and most direct impact of the 2012 USPSTF recommendation was on PCa screening rates. Studies using various metrics have shown a decrease in PSA screening rates after 2012.14-16 PCa screening is largely carried out by primary care providers (PCP) and urologists. A 2016 study by Zavaski et al.16 used the National Ambulatory Medical Care Survey (NAMCS) and compared the rates of PSA screening between 2010 and 2012 for PCP and urologists.16 PCP saw a decrease in PSA screen rates from 36.4% to 16.4% (adjusted odds ratio: 0.44; 95% CI: 0.24–0.80). Urologists, who rely less heavily on the USPSTF, saw a decrease in PSA screening rates from 38.7% to 34.5% (adjusted odds ratio: 0.89; 95% CI: 0.19–1.84). Two large population studies showed a significant decrease in PSA screening after 2012. A 2015 study by Drazer et al.17 looked at data from the National Health Interview Survey (NHIS) and showed a decline in PSA screening between 2010 and 2013.17 Rates declined for males aged 50–59 years (33.2% to 24.8%, p<0.01), aged 60-74 years (51.2% to 43.6%, p<0.01), and aged ≥75 years (43.9% to 37.1%, p=0.03).
This significant nationwide decrease was also seen in a 2016 study by Jemal et al.15 using Surveillance, Epidemiology, and End Results (SEER) data, which showed an 18% decrease in screening rates between 2010 and 2013. The decrease in PCa was greatest among males aged 55–74 years.
Consistent with a decrease in PCa screening rates following the 2012 USPSTF recommendations, studies have shown a decrease in both prostate biopsy rates and incidence of PCa.18-21 A 2017 study by Halpern et al.21 evaluating the number of procedures performed by urologists in the USA from 2009 to 2016 found that the number of prostate biopsies decreased by 28.7% after 2012 (parameter estimate: -0.25; standard error: 0.03; p<0.001).21 Barocas et al.18 analysed the National Cancer Database (NCDB) comparing PCa incidence in 2010 and 2012, and found a 28% decrease in the incident diagnosis of PCa the year after the release of the USPSTF draft.18 A 2019 analysis by Butler et al.19 of the SEER database comparing PCa incidence rates between 2010 and 2015 showed a decrease in the incidence (per 100,000 people) of localised prostate from 195.4 to 131.9 (p<0.001) among males aged 50–74 years and from 189.0 to 123.4 (p<0.001) among males aged ≥75 years.19 However, the decline in PCa incidence has not necessarily been uniform over all risk groups.
A 2019 analysis of the NCD by Fletcher et al.,20 looking at males with clinically localised PCa (T1–4, N0, M0) from 2004 to 2014, showed a shift towards higher-risk PCa.18 They found that among 755,567 males diagnosed with PCa, low-risk PCa decreased (38.32% to 27.23%, p<0.001), while increases were seen in intermediate-risk (40.49% to 46.70%, p<0.001) and high-risk (21.19% to 26.05%, p<0.001). The increase in intermediate and high-risk PCa were largely because of an increase in Gleason Score. Several studies have shown an increase in Gleason Score and a general higher-risk, more aggressive PCa following the 2012 recommendations.19,20,22
Most concerning, numerous studies have documented an increase in the rate of metastatic PCa.19,20,22,23 The 2019 SEER analysis by Butler et al.19 showed an increase in the incidence of metastatic PCa from 6.2 to 7.1 (p<0.001).19 Using SEER data, Kelly et al.23 further analysed the change in metastatic PCa.23 The authors found that metastatic PCa declined by 1.45% per year from 2004 to 2007 and then increased by 0.58% per year after 2008 and 2.40% per year after 2012. They had forecasted that metastatic PCa would continue to increase by 1.03% per year through to 2025, leading to an increase in the annual burden by 42%. It remains to be seen if the increasing aggressiveness, risk group, and rate of metastatic disease translates into a decrease in survival.
CRITICISM OF 2012 USPSTF RECOMMENDATIONS AND 2018 UPDATE
Several criticisms exist regarding the studies used to guide the 2012 USPSTF recommendation against PSA-based PCa screening. The PLCO trial has been considered highly flawed and not a true comparison of screening versus not screening, as a high number of males were screened regardless of randomisation group and a low number of males in screening groups received biopsies. Specifically, 44% of all those enrolled had at least one PSA screen before enrolment, 40–52% in the control group were offered PSA screening per year (resulting in 79% having had at least one PSA screen at some point in the trial), and only 41% of males in the screening arm received biopsy following a positive PSA result.11,24 A chief concern in the use of the ERSPC results is that, at the time of the 2012 USPSTF analysis, only 9 years of follow-up had been reported.13 Given that PCa is a highly indolent disease and factoring in 11–12 years of lead time bias because of screening, 9 years may have been too short an interval to truly gauge the impact of PSA screening.4,25 Subsequent follow-ups of the ERSPC trial have shown continued improvement with time.26,27
Following the 2012 recommendations, the USPSTF conducted a review of studies published between July 2011 and February 2018.28 Sixty-three studies in 104 publications were included in their analysis. Of the studies evaluated, three were randomised controlled trials: the PLCO trial, the ESRCP trial (13-year update), and the Cluster randomised trial of PSA testing for prostate cancer (CAP). These three trials largely constituted the studies used to gauge the impact of PSA screening. The PLCO trial and the CAP trial showed no benefit to PSA screening. The ESRCP update showed a reduction in the incidence of metastatic PCa (relative risk: 0.7; 95% CI: 0.6–0.82) and a reduction in the number needed to screen to prevent one death from PCa to 781.
Based on the continued literature review, the USPSTF released updated PCa screening recommendations in 2018.29 They continued to recommend against PSA-based screening for males aged ≥70 years (Grade D). However, for males aged 55–69 years, they advised PSA-based screening based on a shared decision-making approach between physician and patient. The USPSTF continued to note the potential harms of PSA screening (false positives, side effects of biopsy, over diagnosis, and side effects of treatment). The improvement in prevention of PCa deaths and in development of metastatic disease seen in the ESRCP trial now warranted consideration of screening. Specifically, that the decision to screen should be an individual one after being informed of the risk and the benefits of screening (Grade C).
The 2018 updates also reviewed and discussed the role of screening in African American males and those with family history of PCa.29 They noted that the two groups, particularly African American males, had an increased risk of PCa that might further increase the benefits of PSA-based PCa screening. Unfortunately, existing data were insufficient to fully evaluate PSA-based screening in these specific subgroups. The ESRCP trial did not record data for race but given the comparatively low percentage of those of African descent in Europe, this likely means that the group was not well represented.
The ERSPC trial continues to show increasing benefit to PSA-based PCa screening. In 2019, a 16-year update was published, which showed a further reduction in the number needed to screen to prevent one PCa death to 570.30 This is within numbers needed to screen that are observed with other recommended cancer screening tests, including those for breast cancer and colon cancer. It should be noted that although the median follow-up time in the ESRCP trial was 15.5 years from randomisation, the median follow-up time from PCa diagnosis was only 8.8 years in the screening group and 5.4 years in the control group.29 Future updates may likely continue to show increasing benefit to PCa screening. Given their greater risk of aggressive PCa, the benefits seen in the ESRCP trial may indicate an even greater potential benefit in African Americans.
OTHER AGENCY GUIDELINES: AMERICAN UROLOGICAL ASSOCIATION AND EUROPEAN ASSOCIATION OF UROLOGY RECOMMENDATIONS
The USPSTF recommendations are influential, but other agencies also issue PCa screening guidelines. Following the 2012 USPSTF recommendation, the American Urological Association (AUA) released guidelines in 2013, which were reviewed and reaffirmed in 2018, recommending that males aged 55–69 years be offered PSA screening based on shared decision-making. Furthermore, they recommended against routine screening in males aged 40–54 years, but noted it may be considered for high-risk patients.31 The European Association of Urology (EAU) has issued similar guidelines recommending a risk-adaptive, shared decision-making approach for average-risk males aged >50 years and high-risk males aged >45 years.32Overall, many agencies are transitioning to an individualised, shared decision-making approach. Table 1 summarises various agencies and their associated PCa screening guidelines.
PROSTATE CANCER SCREENING FOR AFRICAN AMERICANS OR MALES OF AFRICAN DESCENT
African American males are more likely to develop PCa, develop it at a younger age, and are twice as likely to die from PCa.29 These differences can be attributed to differences in the genetic make-up of the cancer,33reduced access to screening34 or inadequate follow-up after screening,35 or difference in treatment.36 The USPSTF bases its recommendation on three randomised controlled trials in which the African American males were under-represented, for example, with 4% in the PLCO trial.16,29,37 These trials were not enough to determine if there any different screenings needed for African American males.29 Potential risk of harm was higher for African American males compared with that for Caucasian males, reported an analysis of the PLCO trial.38 Given the higher incidence and mortality among African American males, this population is more likely to benefit from PSA screening than the general population, but further studies are warranted to confirm this hypothesis. The USPSTF currently does not recommend more aggressive screening for African American males because of insufficient evidence; however, they believe that a reasonable approach would be for physicians to inform their patients who are African American about their increased risk and potential benefits and harms of screening, thus helping to make an informed, personalised decision about PCa screening.
AFRICAN AMERICAN-SPECIFIC PROSTATE CANCER SCREENING GUIDELINES: A SURVEY OF SPECIALISTS
PCa in African American males has several unique epidemiologic, genetic, and clinical features compared to PCa in Caucasian males, so it may have been inappropriate to apply the 2012
USPSTF PCa screening guidelines within the African American population. The authors had previously hypothesised that the higher mortality associated with PCa among African American males might be reduced by designing a race-specific screening schema. The authors then conducted a survey that would poll a panel of PCa specialists about various options available to develop a unique screening guideline for African American males. Their responses and opinions were used to propose a novel PCa screening guideline for African American males.
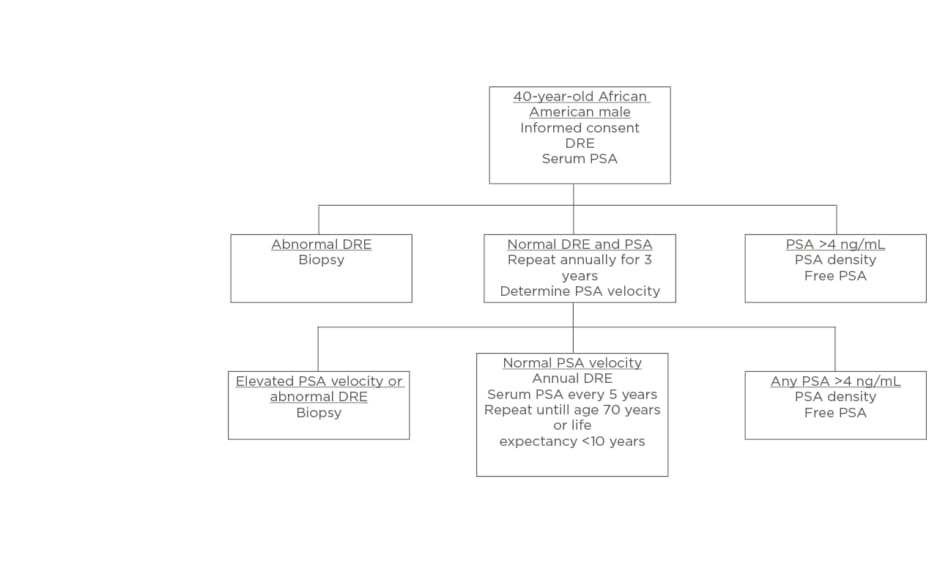
The majority of surveyed PCa specialists believed African American males should be screened using distinct PCa screening. Using input from the expert panel, the authors presented a unique screening protocol for African American males. The presurvey schematic, shown in Figure 1, was modified using the results of the survey to produce a schema, which more closely aligns to the views of the expert panel, shown in Figure 2.
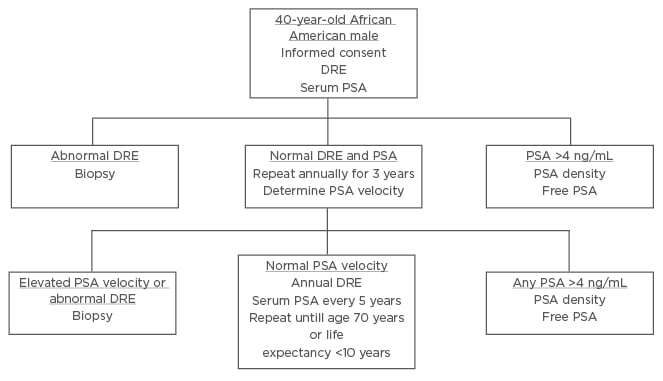
Figure 1: Screening schematic for African American males proposed prior to interviewing experts.
DRE: digital rectal examination; PSA: prostate specific antigen.
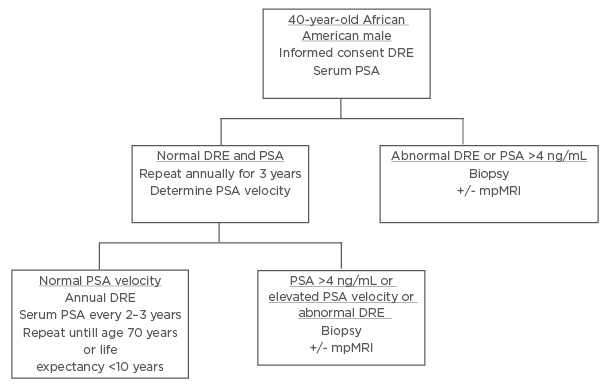
Figure 2: Revised screening schematic for African American males after interviewing experts.
PSA: Prostate Specific Antigen; DRE: digital rectal examination; mpMRI: multiparametric MRI.
Three notable modifications were made: at the initial screening (at 40 years of age) in the setting of a serum PSA >4 ng/mL, patients would proceed directly to biopsy rather than PSA density or free PSA; during annual digital rectal examination and PSA tests, if any PSA is reported as >4 ng/mL, patients are to proceed directly to biopsy; thirdly, in the setting of normal PSA velocity, serum PSA should be measured every 2–3 years, rather than at 5-year intervals.
This study was limited by a low response rate and had potential for confounding bias. Physicians who stand to benefit (financially, for example) from additional screening have an inherent conflict of interest.
A multivariate analysis should have been performed to control for this bias and provide additional information about the opinions expressed in the survey (Vijayakumar, personal communication).
The majority of surveyed PCa specialists believed that separate PCa screening guidelines for African American males should exist because of their unique epidemiological, genetic, social, and clinical features. The authors constructed and presented a novel screening protocol for African American males using the input from the expert panel. Considering the USPSTF release of updated PCa screening guidelines that encourage physicians and patients to make an informed discussion about PCa screening, the timing is appropriate to introduce specific screening guidelines for African American males.
THE ROLE OF MRI IN PROSTATE CANCER SCREENING
PCa is a common, serious disease for which outcomes improve if caught earlier in its course. Unfortunately, the task of screening is complicated by the large prevalence of low-grade disease. The history of PCa screening has been marred by test results that have led to unnecessary fears and procedures in patients whose disease would likely have never become problematic.9New and improved screening guidelines will need to look to new modalities to rightly discern between indolent and sinister disease, and MRI may be able to play a vital role if its current limitations can be overcome. Unfortunately, much of the data on the ability of MRI to screen for PCa is extrapolated from patients known to have elevated PSA levels, but that will soon to change.39 There is an ongoing randomised controlled trial comparing multiparametric-MRI-aided screening guidelines to those using PSA, which will achieve results in June 2020. The potential advantages of MRI-aided screening guidelines are three-fold. First, from available data, multiparametric-MRI appears to have a high negative predictive value in populations of low cancer incidence, which would be maximised in a screening setting.40 Secondly, there is potential for MRI to preferentially detect high-grade disease as it has been shown to be less sensitive in low-grade disease.41 Thirdly, prospective data have shown that biopsy of a lesion detected by MRI has a much higher negative predictive value than standard transrectal ultrasound-guided biopsy.42 There seem to be two dominant limitations of using MRI in a screening schema: inter-reader variability and cost. A study by Branger43 showed that a negative MRI could not reliably exclude Gleason pattern 4 disease or extracapsular extension, but in the hands of an experienced radiologist, disease of core length ≥5 mm or Gleason Score ≥7 negative predictive values exceeded 95%. On an individual level, the cost of a prostate MRI is similar to the cost of a screening colonoscopy.44 However, there may be an overall cost advantage when considering the potential system-wide reduction in unnecessary biopsies and treatments. In the event that multiparametric-MRI is shown to be superior to PSA-based guidelines, economic studies will be required. In conclusion, MRI-based PCa screening has potential to offer a more appropriate screening measure than current methods, but its utility and cost-effectiveness must be demonstrated first.
COST–BENEFIT ANALYSIS
Several different strategies have been utilised to determine cost-effectiveness of PSA screening. These include, but are not limited to, no screening, biennial screening from age 40–74 years, a single screening at age 60 years, screening every 4 years from age 55–69 years, and screening every 4 years from age 50–74 years. In a Canadian study, Pataky et al.45 studied the cost-effectiveness of PSA screening using an existing model of PCa. They determined that PCa mortality reductions occurred with 4-year PSA screening of 55–69-year-old males, as well as with 2-year screenings of those aged 40–74 years. However, this model also projected an increase in overdiagnosis with either
screening strategy.45
In a microsimulation model, it was found that out of a population of 1,000 males in a nonscreening scenario, there were 136 cases of PCa diagnosis, 35 deaths, and 246 negative biopsies.
However, in the population for the screening scenario of 1,000 males, the model showed 178 diagnoses of PCa and 27 deaths, with 443 negative biopsies. In this model, it was predicted that PSA screening every 4 years increased the cost for PCa by 44%, with eight fewer deaths per 1,000 males.46 Although the cost of a PSA screening is $30–100 USD, the vast majority of the cost of PCa can be accredited to diagnosis and treatment. The rate of false positive PSA screenings leads to an increase in unnecessary diagnostic biopsies and the associated risk of undergoing these procedures.47 There is a need for better PCa screening that will reduce the number of false positives, increase the number of true positives, and greatly reduce the cost of diagnosing and treating PCa.
FAMILIAL HISTORY AND THE RISK OF PROSTATE CANCER INCIDENCE AND SEVERITY
Factors that have been identified as familial risks for a PCa include being a male of African American descent, having a first-degree relative with a history of PCa, and advancing age. Using the Swedish Family-Cancer Database, it was found that males who had a father, brothers, or both, with a history of PCa were at an increased incidence of developing the disease.48 Further, Barber et al.49 determined that males with a family history of either breast cancer or PCa had an increased risk of PCa.49 Family history of PCa alone was associated with a 68% increased risk of total disease and a 72% increased risk of lethal disease.49 From a systematic review and meta-analysis, Telang et al.50 found no increased risk of PCa progression in males with a family history of PCa.50 Although an increased incidence of PCa is found in males with first-degree familial history of PCa, this does appear to cause an increase in the incidence of cancer progression in these individuals. However, Bratt et al.,51 in a nationwide population-based study in the USA, found age-specific PCa incidences were much higher among males with family history of PCa and of high-risk cancer. It appears that the risk of PCa is 2–3 times higher among those with a family history of PCa and those cancers that can lead to mortality from PCa.
SUMMARY, CONCLUSIONS, AND RECOMMENDATIONS
The authors hope this review will bring some clarity to the issues associated with PCa screening in Western nations. The issues associated with PCa screening in less developed countries are beyond the scope of this review. The following can be summarised:
- In trying to balance between early diagnosis and avoiding unnecessary procedures or cost, the pendulum, in the past decade, has swung to the side of excessive avoidance of PCa screening. This has led to worsening of outcomes in PCa. This fact has been realised in recent years and efforts to correct this trend is underway. This review is part of that process.
- PSA-based PCa screening is inadequate because of its lack of specificity; however, this may be the only option that is acceptable and available. The authors have come up with processes that may help overcome some of the shortcomings of PSA-based PCa screening (Figure 1 and Figure 2).
- New biomarkers and molecular markers have shown promise in overcoming the defects of PSA-based screening in PCa (Table 2). However, the usefulness of each need to be robustly compared so that uniform guidelines for post-PSA-era PCa screening can evolve. Pharmaceutical and other private entities have no incentives to conduct clinical trials that would compare different commercial panels against each other. The National Cancer Institute (NCI)/National Institutes of Health (NIH), the National Health Service (NHS), and similar national health research or health agencies have to design new clinical trials to address this aspect of the PCa screening.
- Special populations, such as African Americans, the elderly, or those with familial history of PCa, require special care and more specific, and perhaps separate, guidelines. The authors attempted such recommendations in this paper. These attempts should be considered a work in progress, and mean to act as catalyst for new clinical trials and to fill a void until post-PSA-era guidelines evolve.
- Policy makers are urged to develop new clinical trials quickly to address the deficits related to the current state of PCa screening highlighted in this review paper. This will help save lives now and in the future, especially for special populations. These clinical trials should aim to balance the goals of saving lives from PCa mortality, avoiding unnecessary interventions and treatment, unnecessary cost associated with unnecessary interventions, and the outcome endpoints should include quality-of-life improvements. The clinical trials being designed should have sufficient power to address the issues related to the special populations.
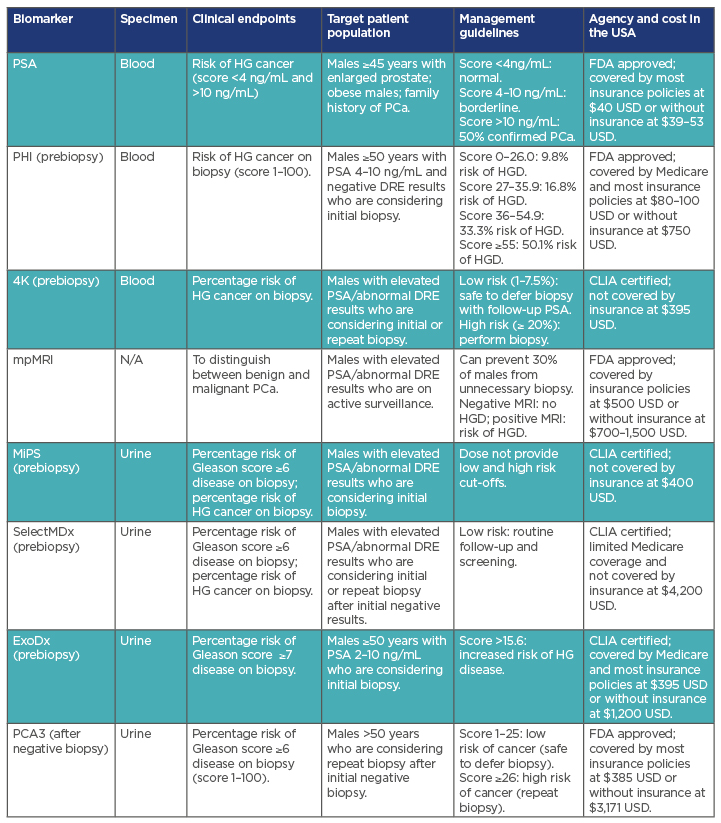
Table 2: Summary of screening biomarkers in prostate cancer management.
4K: Prostate Specific Kallikrein; CLIA: Clinical Laboratory Improvement Amendments; DRE: digital rectal examination; ExoDx: Intelliscore, nondigital digital rectal examination; FDA: U.S. Food and Drug Administration; HG: high grade (Gleason ≥7); HGD: high-grade disease; MiPS: Mi Prostate Score Urine Test; mpMRI: multiparametric MRI; PCA3: prostate cancer antigen 3 test; PHI: Prostate health index; SelectMDx: liquid biopsy.