Abstract
Renal arteriovenous malformations and fistula are an uncommon, underdiagnosed condition that can be asymptomatic. However, there is a real risk of rupture and severe bleeding. Imaging techniques have a critical role in planning the treatment. Arteriography is the gold standard but is invasive. Diagnostic selective arteriography can be followed by embolisation of the lesion during the same procedure. Although invasive, because of the potential risk for rupture, arteriography is the elective technique when intervention is planned.
We report a rare case of an adult male patient incidentally diagnosed with arteriovenous malformation. Since he had no prior history of renal intervention or trauma, a diagnosis of idiopathic renal arteriovenous malformation was made. We describe the computed tomography findings and management outcome. This asymptomatic, though potentially lethal, condition can be treated with minimally invasive methods.
INTRODUCTION
Renal arteriovenous malformation (RAVM) is a rare entity, with an estimated prevalence of <0.04%.1 Acquired communications between renal arteries and veins are defined as arteriovenous fistulas (RAVF) and are usually secondary to needle biopsy of the kidney or trauma. Conversely, arteriovenous malformations are rare congenital or idiopathic malformations.2 Cho and Stanley1 classified the malformations into three types according to angiographic morphology: Type I, a single or a few arteries (fewer than four) shunted into a single draining vein; Type II, multiple arterioles shunted into a single draining vein; and Type III, multiple shunts between the arterioles and venules, forming a complex vascular network. Type III can be further subdivided into two types, according to the size of the fistulous channels (IIIa, nondilated; IIIb, dilated).1
The malformations are classified as congenital, acquired, and idiopathic,3 or intra and extrarenal.4 More than 70–75% are acquired, caused by trauma, malignancy, inflammation, or iatrogenesis and consist of a single, tortuous dilated artery communicating with a vein as an aneurysmal fistula (RAVF).5 About 14–27% of cases are congenital and 2.8% are considered idiopathic (RAVM). The latter are probably caused by fibromuscular dysplasia, which results in arteriovenous communication.6 Angiographic features have been traditionally used to classify them as either cirsoid or aneurysmal type. The cirsoid type has a knotted, tortuous appearance comprising numerous vessels and multiple arteriovenous (AV) interconnections. The aneurysmal type is a direct RAVF without nidus-like components, which are often associated with aneurysmal dilatation of the feeding artery and/or draining veins.7 In general, congenital lesions typically have a cirsoid appearance, and acquired or idiopathic lesions are usually aneurysmal.8
We report a rare case of an asymptomatic, adult male patient diagnosed with a RAVM, describing the computed tomography (CT) findings and management outcome.
CASE REPORT
A 43-year-old male presented with a urinary tract infection and lower urinary tract symptoms after drainage of a perineal abscess. There was no haematuria, no hypertension, and renal function was normal with a serum creatinine of 0.88 mg/dL and a glomerular filtration rate of >90 mL/min/1.73m2. Medical history contained severe haemophilia Type A (Factor VIII <1%).
He had a 39°C fever. Urine cultures showed growth of Escherichia coli and Proteus species, both sensitive to quinolones. After treatment with antibiotics, his complaints disappeared. There was no pneumaturia nor transanal urinary leakage. Rectal examination was normal. The urinary system ultrasound examination revealed however, a large anechoic cavity located in the right renal pelvis sprouting in different calices (Figure 1). The patient did not report any history of renal trauma or recent medical intervention in which percutaneous instrumentation was used.
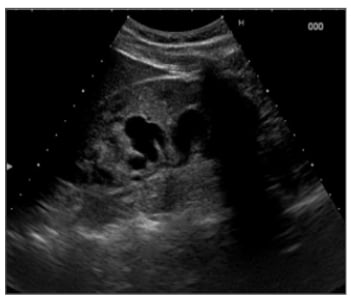
Figure 1: Ultrasound showing an anechoic cavity in the right renal kidney.
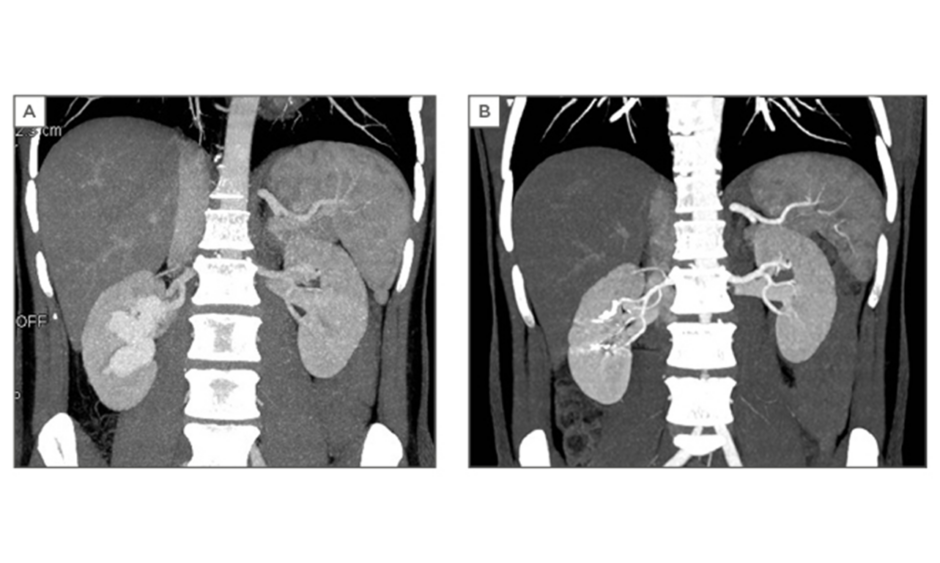
On colour Doppler ultrasound, the lesion showed turbulent high blood flow. The patient underwent a CT angiography (Lightspeed VCT 64-Slice, GE Healthcare, Chicago, Illinois, USA). An unenhanced CT was performed of the total abdomen. We started the arterial phase of the kidneys at 100 HU. We administered 120 cc of contrast (Xenetix 350/water) at a flow rate of 3 cc/seconds followed by 20 cc of water at a flow rate of 2 cc/seconds. Then we performed the venous phase of the kidneys and after 300 seconds a delayed phase of the abdomen was performed. The CT angiography demonstrated an aneurysmal type of arteriovenous malformation in the right kidney (Figure 2A).
Because of the size of the malformation and with his medical history of haemophilia in mind, we decided to carry the patient immediately into the angiography room for endovascular treatment (Allura Xper FD20/10, Phillips, Amsterdam, Netherlands). This procedure in elective conditions has less risk of major bleeding than in an urgent setting.
Selective right renal artery angiography was performed using a 5 Fr 11 cm Arrow-Flex® sheath (Teleflex Incorporated, Wayne, Pennsylvania, USA) with a 0.035 inch Terumo Angled guidewire coupled with a 5 Fr Cobra Glidecatheter (Terumo Corporation, Tokyo, Japan). The lesion was selectively catheterised with a microcatheter (Progreat 2,7F, 0,0025” Terumo Corporation) and embolised with microcoils (Tornado® MWCE-18S-7/3, 18S-8/5, 18S-6/2, 18S-5/2 and18S-10/4, Soft Platinum MWCE-18S-5.2-12-B, Cook Medical, Bloomington,
Indiana, USA; AZUR Hydrocoil-18 D2 L20 45-280202, Terumo Corporation). The embolisation was successfully performed (Figure 3A and 3B). The patient’s haemodynamic parameters, such as blood pressure, were monitored. He was discharged the following day. Postoperative follow-up was uneventful. The kidney function was still preserved with a serum creatinine of 0.82 mg/dL and a glomerular filtration rate of >90 mL/min/1.73m2 and CT angiography (Figure 2B) showed no persistent lesion.
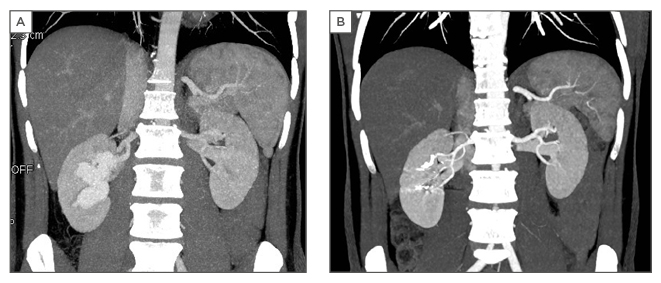
Figure 2: A) Computed tomography angiography reveals the arteriovenous malformation; B) Postoperative computed tomography angiography.
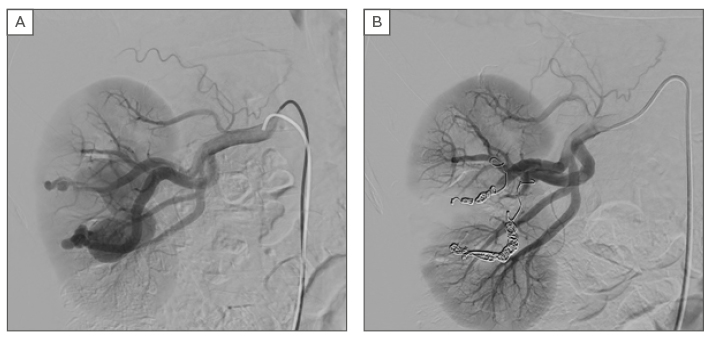
Figure 3: A) Selective arteriography of the right renal artery reveals the arteriovenous malformation; B) Result after selective embolisation of the arteriovenous malformation.
DISCUSSION
RAVMs represent an abnormal communication between the renal arterial and venous system.9 A malformation is usually located in the collecting system and not in the renal parenchyma, with most of the cases on the kidney upper pole (45%), but it also can be detected in the mid-point or in the kidney lower pole in an equal ratio.10 The left kidney is more frequently involved, and women are affected twice as often as men. The peak incidence is in patients aged 30–40 years, and RAVMs are rare in the paediatric population.11 The first intrarenal fistula was reported by Vorela in 1928.4 Surgery, whether nephrectomy or ligation of feeding vessels, has long been the standard treatment for symptomatic arteriovenous malformations or fistulas. The current therapeutic goal for RAVF is to cure arteriovenous shunting but preserve most or all kidney function on the involved side.5 As an alternative to surgery, percutaneous procedures are becoming widely accepted.12,13
RAVMs can be asymptomatic, but may manifest with haematuria, flank pain, flank bruit, hypertension, perinephric haematoma, and high-output heart failure.7 Although our patient was recently diagnosed with a urinary tract infection after perineal abscess drainage, we believe this infection is related to the intervention rather than caused by the malformation and so he can be classified as an asymptomatic case.
A search of the English language literature using the MEDLINE® database via PubMed revealed only a few asymptomatic cases of RAVM during the last 10 years (Table 1). We excluded all symptomatic cases such as haematuria, hypertension, heart failure, renal failure, or flank pain, and the cases with prior history of renal intervention.
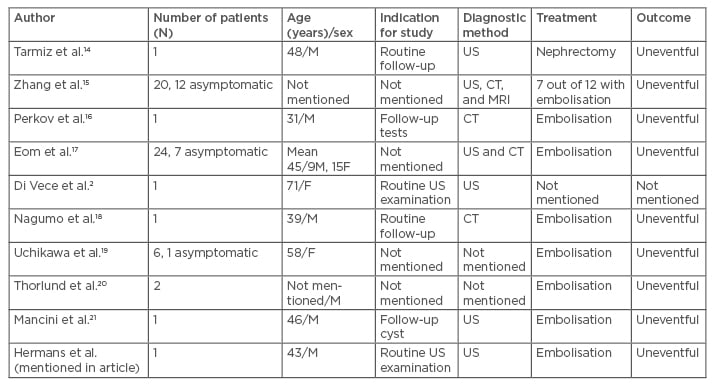
Table 1: Cases of asymptomatic renal arteriovenous malformation.
F: female; M: male; CT: computed tomography; US: ultrasound; MRI: magnetic resonance imaging.
Treatment consists of surgical excision or less invasive intravascular procedures. Surgical treatment (such as partial nephrectomy, total nephrectomy) renal auto-transplantation or vessel ligation, is recommended for malformations of >2cm in diameter in women of childbearing age, with an expanding size on serial angiograms, with an incomplete ring like calcification on radiography, in the presence of uncontrollable hypertension, or in the presence of high output heart failure.22 This hypertension is renin-mediated hypertension caused by fistula-related relative ischaemia; the high-output cardiac failure can be explained by an increase in venous return.23 In order to avoid nephrectomy, reduce preoperative risk, and shorten hospital stay, transarterial embolisation may be regarded as the first-line treatment of RAVM.10
There exist several embolic materials, including particles (gelatine sponge particles and polyvinyl alcohol particles), coils (pushable and detachable coils), vascular plugs, detachable balloons, and liquid materials (absolute ethanol, N-butyl-2-cyanoacrylate, and ethylene vinyl alcohol copolymer [Onyx®]). The appropriate selection of the embolic material is based on the type of RAVMs and the size and flow of the lesion.7 Embolisation of high-flow RAVMs can be technically difficult because the torrential flow through these lesions increases the risk of loss of the embolic device into the pulmonary circulation.24
Transcatheter arterial embolisation may sometimes cause post-embolisation syndrome, which consists of fever, loin pain, nausea, and vomiting. Selective embolisation of RAVMs preserves the renal parenchyma and therefore leads to minimal post-embolisation syndrome.25 Furthermore, there is a risk of recanalisation and/or incomplete occlusion after inadequate or incomplete embolisation.7 The risk of recanalisation depends on the type of embolic material used and the size of the lesion.7 Our patient showed no signs of complications related to the procedure.
Estimation of RAVM instability is difficult or impossible to be detected. There are computer methods that provide support to surgeons to overcome clinical diagnostic limits and help them during the process of medical decision making between invasive or endovascular intervention.9
Our patient met the typical diagnostic criteria to treat with an endovascular procedure. Our aim was to treat the RAVM before it became symptomatic. In consideration of the known haematologic medical history, treatment was planned relatively fast after diagnosis, in particular to avoid bleeding and haematuria. The RAVM was coiled successfully. The postoperative period was uneventful, and renal function was preserved. Yearly follow-up with ultrasound will be provided.
CONCLUSION
RAVMs are a rare entity. We presented a case of idiopathic, asymptomatic AV-malformation in a 43-year-old male who underwent an urgent but elective embolisation. There are only a few asymptomatic cases in the literature who were treated. Even though still asymptomatic, treatment must be considered in the light of newly developing, less invasive, endovascular procedures. The general condition of the patient and his or her symptoms must be considered in the therapeutic decisionmaking. In the past, there were only invasive therapeutic options, but embolisation by selective catheterisation can be considered safe and effective in both elective and urgent cases.