Abstract
Urothelial carcinoma (UC) is the second most common urologic malignancy after prostatic adenocarcinoma. UC comprises more than 90% of urinary bladder tumours. The intense research involving the different molecular aspects of bladder malignancies offers potential opportunities to improve understanding of bladder cancer biology; helps to identify disease earlier; and improves prediction of outcomes or helps targeted therapy.
This review highlights the general concepts of the molecular features: molecular pathways for bladder carcinomas and molecular biomarkers for potential target for treatment of UC of the bladder. This discussion could improve the understating of pathogenesis as well as will provide new therapeutic modules, e.g., targeted therapy.
This article is a review of bladder cancer genetics, focusing on molecular changes and their significance in the pathogenesis and progression of muscle invasive UC. Also, the relevant genetic biomarkers and their products, and new therapeutic targets and agents that are being developed are presented here.
Key Points
1. Molecular technologies have facilitated the identification of bladder cancer genetics and molecular changes, and their significance in the pathogenesis and progression of muscle invasive urothelial carcinoma. The relevant genetic biomarkers and their products, new therapeutic targets and agents, and potential future perspectives are discussed in this article.
2. Biomarkers such as serum vascular endothelial growth factor, circulating tumour cells, and defects in DNA damage repair genes can predict responses to cisplatin-based neoadjuvant chemotherapy in many patients with muscle invasive bladder cancer.
3. High tumour mutational burden has been associated with response to immune checkpoint inhibitors in a metastatic bladder cancer setting. Immune checkpoint inhibitors in the neoadjuvant setting, using pembrolizumab or atezolizumab before radical cystectomy in patients with muscle invasive bladder cancer, is an approach that is in continuous evolution and needs frequent updates.
INTRODUCTION
Bladder cancer (BC) is the fourth most common malignancy in males and shows high prevalence and mortality rates worldwide despite improvements in its management.1,2 Although non-muscle invasive BC is the commonest type, 25–30% of bladder tumours are muscle invasive (MIBC) at the time of diagnosis, and these patients often have a poor prognosis despite traditional treatments.3 Therefore, both in treatment and follow-up, BC remains a challenging disease for urologists. Identifying promising molecular markers and improving the clinical strategies for managing BC have become crucial.4
Histopathological and molecular studies indicate that urothelial carcinomas (UC) follow two different molecular pathways with distinct biological behaviour. UC has two subtypes. One is the papillary, low-grade, non-invasive UC (70%), arising from urothelial papilloma or hyperplasia and have high recurrence rate. The second subtype is muscle invasive UC (Stages pT2–pT4) that often develops metastases, and 5-year survival rate is <50%. They usually arise through the sequences of events: normal to dysplasia to carcinoma in situ to invasive tumours.5,6 Previously, management of UC was based on conventional histologic parameters. However, similar UCs may show different response to treatment, which is the evidence of molecular heterogeneity among histologically similar tumours. Intensive molecular research over the last few decades have provided great insight into the biology of UC. Molecular technologies have facilitated the identification of molecular pathways and predicted outcomes of BC, thereby potentially improving life expectancy of patients.7,8
MOLECULAR BASIS OF CARCINOGENESIS IN ADVANCED BLADDER UROTHELIAL CARCINOMA
The molecular basis of carcinogenesis in advanced bladder UC includes: self-sufficiency in growth (epidermal growth factor receptor [EGFR] and hepatocyte growth factor); insensitivity to inhibition of growth (p RB, p53, and p27); evasion of apoptosis (p53, Fas, cluster of differentiation 40, and B cell lymphoma 2 [Bcl-2]); unlimited ability to replicate (telomerase increase); angiogenesis (vascular endothelial growth factor [VEGF], cyclooxygenase-2 [COX-2], platelet-derived growth factor, IL-8, and basic fibroblast growth factor); thrombospondin, angiostatin, and endostatin; tissue invasion (matrix metalloproteinase tissue inhibitor of metalloproteinase); and metastasis (P-cadherin, E-cadherin, β-catenin, and cluster of differentiation 44).
NEW ERA OF MOLECULAR MARKERS AND INVASIVE BLADDER CARCINOMA
Over the last few decades, cisplatin-based chemotherapy has been practiced as the first-line treatment in advanced UC; no potential progress has been evident in the treatment of MIBC. As cisplatin-based chemotherapy is effective in only 30–40% cases of BC, novel therapeutic approaches are crying demand for this lethal cancer.9
New insights into the molecular pathology of BC have focused on some promising therapeutic targets.10 The phosphoinositide 3-kinase (PI3K)/ protein kinase B (Akt)/mammalian target of rapamycin (mTOR) pathway, the mitogen-activated protein kinase pathway, CDKN2A/CDK4/CCND1 and receptor tyrosine kinases/Ras pathways (including ERBB2 [HER2], ERBB3, and FGFR3, as well as chromatin regulatory genes) have critical role in bladder tumourigenesis, specifically in high-grade urothelial carcinomas.11,12 So, further exploration of this pathways in BC is important for prognostic information and targeted therapy.
In the following discussion, UC related proto-oncogenes and tumour suppressor genes and growth factors are emphasised with their therapeutic implication in invasive BC.
TUMOUR SUPPRESSOR GENES
p53
Wild-type p53 promotes anti-cancer activity by inducing cell cycle arrest, apoptosis in response to DNA damage, genomic stability, and inhibition of angiogenesis. On the other hand, the mutant p53 loses its anti-tumour effect and induces abnormal gene expression, thereby leading to tumour progression.13,14 A Phase II clinical trial on the ubiquitin–proteosome proteolytic pathway that regulates metabolism p53 has been conducted by using proteosome inhibitor (bortezomib) in 18 patients with advanced or metastatic UC, and found no effect on its own
Gene therapy by adenoviral vectors on pre-clinical BC cell lines are also on trial to transduce cells for producing the p53 protein; and the preliminary results are promising.15,16
Rb
The Rb gene is responsible for progression of UC. The incidence of Rb mutations is higher in invasive UC (about 37%). There is evidence to support the association between the loss of Rb gene expression and progression of UC in patients with muscle invasive cancer. Pre-clinical trials on adenoviral vectors have also produced optimistic results when investigating transduction of BC cell lines with the Rb gene.17,18
PROTO-ONCOGENES
B cell lymphoma 2
Mutations of proto-oncogenes result in the over-expression of gene product or altered proteins.19 Bcl-2 is a key protein regulator for the cell cycle and apoptotic pathway. Apoptosis is an essential mechanism for radio- and chemotherapy induced cell death. Overexpression of Bcl-2 alters the sensitivity of chemo- and radiotherapy to tumour cells.20,21 Pollack et al.22 analysed 107 patients where only 16 cases were upstaged and 75% (12 cases) of these tested Bcl-2 positive by immunohistochemical analysis. Yet only 24% of those that exhibited Bcl-2 overexpression were upstaged. So, they hypothesised that Bcl-2 overexpression was associated with impaired radiation response in patients with invasive BC treated with pre-operative radiotherapy.22 In BC cell lines, antisense oligonucleotides (oblimersen) down-regulate Bcl-2 expression and enhance apoptosis in response to chemotherapy. The oblimersen Bcl-2 antisense therapy represents a promising new apoptosis-modulating strategy and pre-clinical trials support this synergistic therapeutic role for oblimersen with cytotoxic drugs.23,24
C-erb B-1 and C-erb B-2
C-erb B-1 and C-erb B-2 oncogenes encode transmembrane proteins and EGFR and HER2, respectively.25 Identification of predictive biomarkers of EGFR targeted therapy can improve the development of anti-EGFR drugs. Rebouissou et al.26 reported EGFR as a potential therapeutic target for MIBC displaying basal-like phenotype. Another EGFR inhibitor, gefitinib had shown promising result during clinical trials in combination with gemcitabine and cisplatin.27 Some Phase II/III trails on dual inhibitors of ErbB-1 and ErbB-2 receptor tyrosine kinases such as lapatinib were proven to be well tolerated in patients with EGFR and/or HER2 overexpressing MIBC.28,29
A standardised trail on 1,005 patients with MIBC by Laé et al.30 showed that approximately 5% of MIBC had HER2 gene amplification. There are several ongoing clinical trials to identify potential candidates for targeted therapy in MIBC patients with HER2 overexpression. A Phase II trial has examined the response of trastuzumab (monoclonal antibody) in 59 patients with muscle invasive HER2 over-expressive BC and documented 73% response rate.31 A Phase II trial of 44 cases of HER2 positive UC showed a 70% response when evaluating trastuzumab in combination with chemotherapeutic drugs such as paclitaxel, gemcitabine, and, carboplatin.32
PI3K/Akt/mTOR Pathway
The mTOR pathway plays a vital role in the development UC. This pathway includes upstream activators such as PI3K and Akt, negative regulators such as tuberous sclerosis 1 and 2, and downstream effectors such as p70 S6 kinase and eukaryotic initiation factor 4E. Due to its basic role in tumour growth, researchers have focused on developing targeted therapy on the mTOR.33 Based on durable response in other tumours and pre-clinical trails in BC cell lines, buparlisib (PI3K blockade) is being investigated as a second-line treatment for patients with advanced UC.34 Again, NVP-BEZ235 (dual PI3K/mTOR inhibitors) showed significant anti-tumour effect on cisplatin-resistant BC cells lines, but it can activate mitogen-activated protein kinase/extracellular signal-regulated kinase pathway.35 A Phase II study on 45 patients evaluated the role of everolimus (mTOR inhibitor) in advanced UC, but effective response was documented in only three cases.36
ANGIOGENIC-ANTIANGIOGENIC FACTORS
Vascular Endothelial Growth Factor
VEGF proteins and gene expressions are detected at high levels in high-grade and muscle invasive UCs and are associated with poor survival.37,38 Again, some studies showed that VEGF expression was significantly higher in non-MIBC compared to MIBC as the rate of tumour growth is higher at the early stage of the disease.39,40 So, the inhibition of VEGF transcripts significantly reduces the proliferation rate of the bladder cancer cells41 and blockade of VEGF receptor reduces growth and invasion of bladder cancer cells.42,43 Bevacizumab (VEGF antibody) and ramucirumab (VEGFR2 antibody), used in combination with chemotherapy, showed promising result in Phase II clinical studies of advanced UC. There are multiple ongoing Phase II trials with other agents targeting VEGF receptors, including sunitinib, sorafenib, and pazopanib.44,45
Cyclooxygenase-2
The selective COX-2 inhibitor celecoxib has chemo-protective activity against various cancers, including BC. It inhibits the proliferation, migration, invasion, and epithelial-to-mesenchymal transition of BC cells.46 Epidemiological and pre-clinical evidence suggest that COX-2 inhibitors are promising target for BC. COX-2 expression in the UC is associated with a high grade and an advanced stage, and is an independent predictor of disease progression and survival. However, future trials on COX-2 inhibitors should be tested as a standardised therapy to improve the effectiveness of drugs.47
Thrombospondin-1
Down-regulation of thrombospondin-1 (TSP-1) expression is independently associated with cancer recurrence and mortality. Loss of TSP-1 expression is associated with alterations in other cell cycle regulators such as p21, p53, and p27 expression.48 The newer molecular agents such as the TSP analogue (ABT-510) and TSP-1 mimetics (D-isoleucyl enantiomer TSP-1 heptapeptide) are in Phase II clinical trials, which have been shown to reduce micro-vessel density and increase apoptosis in bladder tumours.49,50
The genes, proteins, and molecules with the potential to alter the muscle invasive UCs with their therapeutic targets are shown in Table 1.
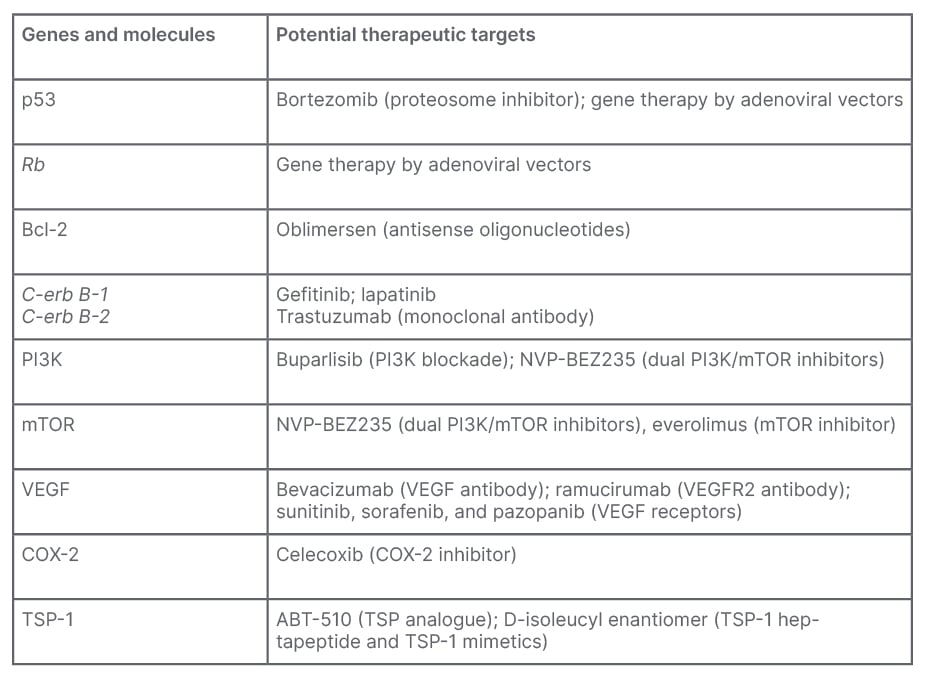
Table 1: Summary of genes and molecules with potential therapeutic targets.
Bcl-2: B cell lymphoma 2; COX-2: cyclooxygenase-2; mTOR: mammalian target of rapamycin; PI3K: phosphoinositide 3-kinase; TSP-1: thrombospondin-1; VEGF: vascular endothelial growth factor.
Some key messages should be pointed out. For instance, many predictive biomarkers were investigated (e.g., serum VEGF, circulating tumour cells, and defects in DNA damage repair genes, which involve ERCC2, ATM, RB1, and FANCC). These biomarkers can predict responses to cisplatin-based neoadjuvant chemotherapy in many patients with MIBC.
In addition, high tumour mutational burden has been associated with response to immune checkpoint inhibitors in metastatic BC. Studies evaluating immune checkpoint inhibitors in the neoadjuvant setting, using pembrolizumab or atezolizumab before radical cystectomy in patients with MIBC (e.g., PURE-01 study and ABACUS study)51 have shown some conflicting results, and, thus, more research is needed. Moreover, programmed death-ligand 1 expression by immunohistochemistry and high tumour mutational burden has demonstrated predictive value in some MIBC settings, but additional studies are merited to explore this topic.
Overall, prognostic and predictive molecular biomarkers will present important adjuncts to current clinical and pathological data. However, large-scale Phase III randomised clinical trials with long-term follow-up are necessary to further investigate this area.
CONCLUSION
Urologists are still treating BC depending on disease stage. Resection of tumour, intravesical mitomycin C, and Bacillus Calmette–Guérin immunotherapy, followed by surveillance are treatment of choice for non-MIBCs. Muscle invasive carcinomas (stage T2) are treated with either radical cystectomy followed by radiotherapy, while metastatic diseases are treated by adjuvant or neoadjuvant chemotherapy. These treatment protocols have improved the disease-free survival; however, overall survival has remained unchanged. Therefore, establishing new treatment regimens for MIBC for better management and overall survival is a crying need. Recent and on-going randomised control trials on molecular biomarkers in muscle invasive disease will be needed to evaluate the precise role and ideal regimen for MIBC.







