BACKGROUND AND AIMS
Accurate prediction of rheumatoid arthritis (RA) development in persons at risk of RA can help to select individuals for early intervention trials. Currently, RA prediction mostly relies on biomarkers such as genetic factors, autoantibodies, and imaging abnormalities, with symptoms being only a minor component.1-3 However, at-risk individuals exhibit a high prevalence of diverse, and often severe, symptoms4,5 and information on the predictive ability of individual symptoms or symptom complexes is still largely lacking. In this prospective cohort study, the authors investigated the predictability of symptoms in persons at risk of RA, using the validated Symptoms in Persons at Risk of Rheumatoid Arthritis (SPARRA) questionnaire.
METHODS AND RESULTS
Individuals from four cohorts from the Netherlands (n=122), UK (n=77), Sweden (n=13), and Switzerland (n=20), were asked to fill out the SPARRA questionnaire, consisting of 69 questions described by van Beers-Tas MH et al.6
Individuals were anticitrullinated protein antibody (ACPA) and/or rheumatoid factor-positive (n=135), had relevant symptoms (arthralgia suspicious for progression to RA) with or without antibodies (n=77), or were first-degree relatives of patients with RA (n=20; excluded from primary analyses). Follow-up was ≥ 24 months. Univariable analyses preselecting possible predictors (Cox regression; p<0.2) were followed by stepwise forward selection (p<0.1) to create a multivariable prediction model. The likelihood ratio test was used to test the added value of the SPARRA items over the clinical prediction model by van de Stadt et al.3
In total, 232 patients were included, 69% were female and the mean (standard deviation) age was 51 years old (13.3). Fifty-eight persons (25%) developed clinical arthritis (n=23, 26, 7, and 2, respectively, in the four groups) after a median of 7 months (interquartile range: 5.3–17.8). In total, 22 SPARRA questions were preselected and entered in the stepwise forward selection procedure. The symptoms that predicted time to development of arthritis are shown in Table 1. The symptom ‘pain that moves from one side to the other’ showed added value to the van de Stadt model in predicting arthritis (likelihood test, p=0.032). The area under the curve of the extended prediction model at 2 years follow-up was 0.73 versus 0.71 (area under the curve van de Stadt model without SPARRA item).
CONCLUSION
Specific symptom details such as pain moving from one side to the other or degree of joint swelling provide useful additional information to estimate a person’s RA risk. The authors are currently creating a shortened version of the SPARRA questionnaire. Its systematic use in prospective at-risk cohorts will enable homogenous symptom data collection which will further improve understanding of the prevalence and predictive ability of greatly diverse symptoms in different at-risk populations.
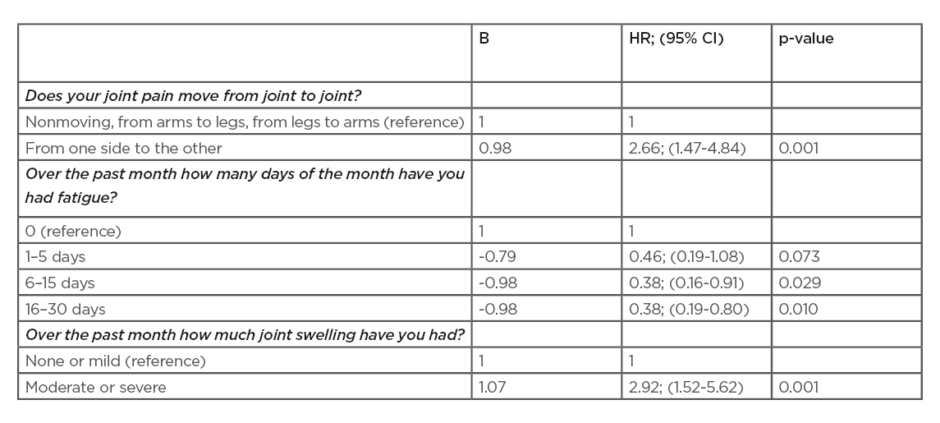
Table 1: Multivariable prediction model of the Symptoms in Persons at Risk of Rheumatoid Arthritis (SPARRA) questions to predict clinical arthritis.
95% CI: 95% confidence interval; HR: Hazard ratio.








